Cavitation in Simulated Cryogenic Liquids
We are currently studying the cavitation of cryogenic fluids through using a refrigerant fluid
which is characterized by high ambient vapor pressure and accessible critical conditions to model
the behavior of liquid hydrogen as it is passing through a low-pressure turbopump. The purpose of
the research is to investigate the impact of the thermodynamic effect - the local cooling of the
liquid around a vaporization zone - to establish cavitation prediction correlations for the
near-critical regime. The test section cross-section is 10x10 cm and the predicted maximum
freestream velocity is 10 m/s. The facility is fitted with a 7.5 kW immersion heater and can
be maintained at absolute pressures of 0.2-5 atm, allowing cavitation at a wide range of
conditions to be studied in several different media. As of May 2007, the water tunnel
facility is operational and delivering pressure spectra and LIF results. The work is now continued by
Sean Kelly.

The water tunnel facility with its 25 hp pump.
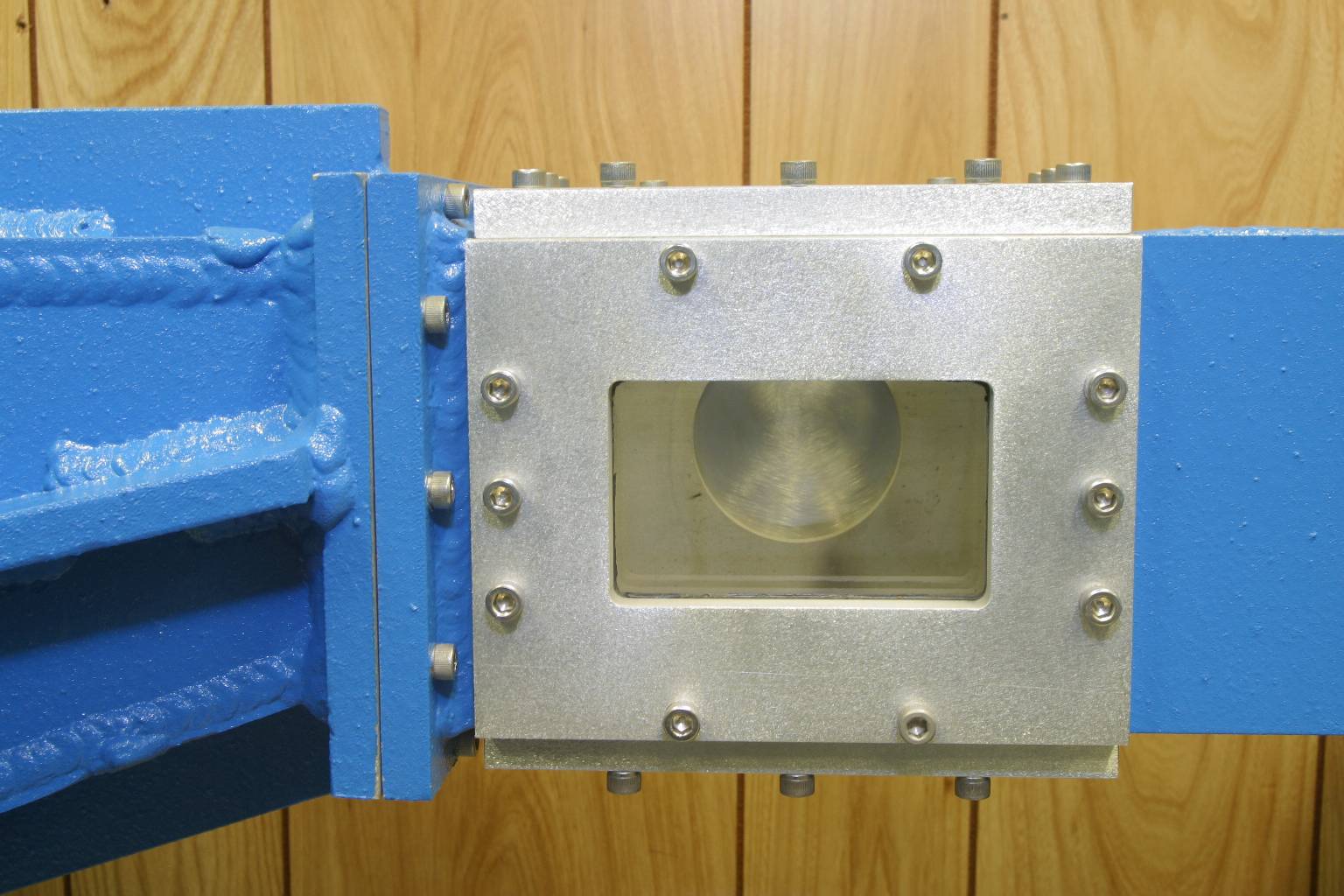
The water tunnel test section with optical access from three directions and the rear access port used for holding the test object, e. g. a hydrofoil.
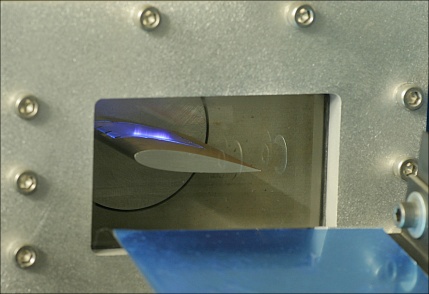
Hydrofoil with transparent cover mounted in the watertunnel. Using a 351 nm laser sheet projecting from the hydrofoil, fluorescence is produced by the fluoroketone.
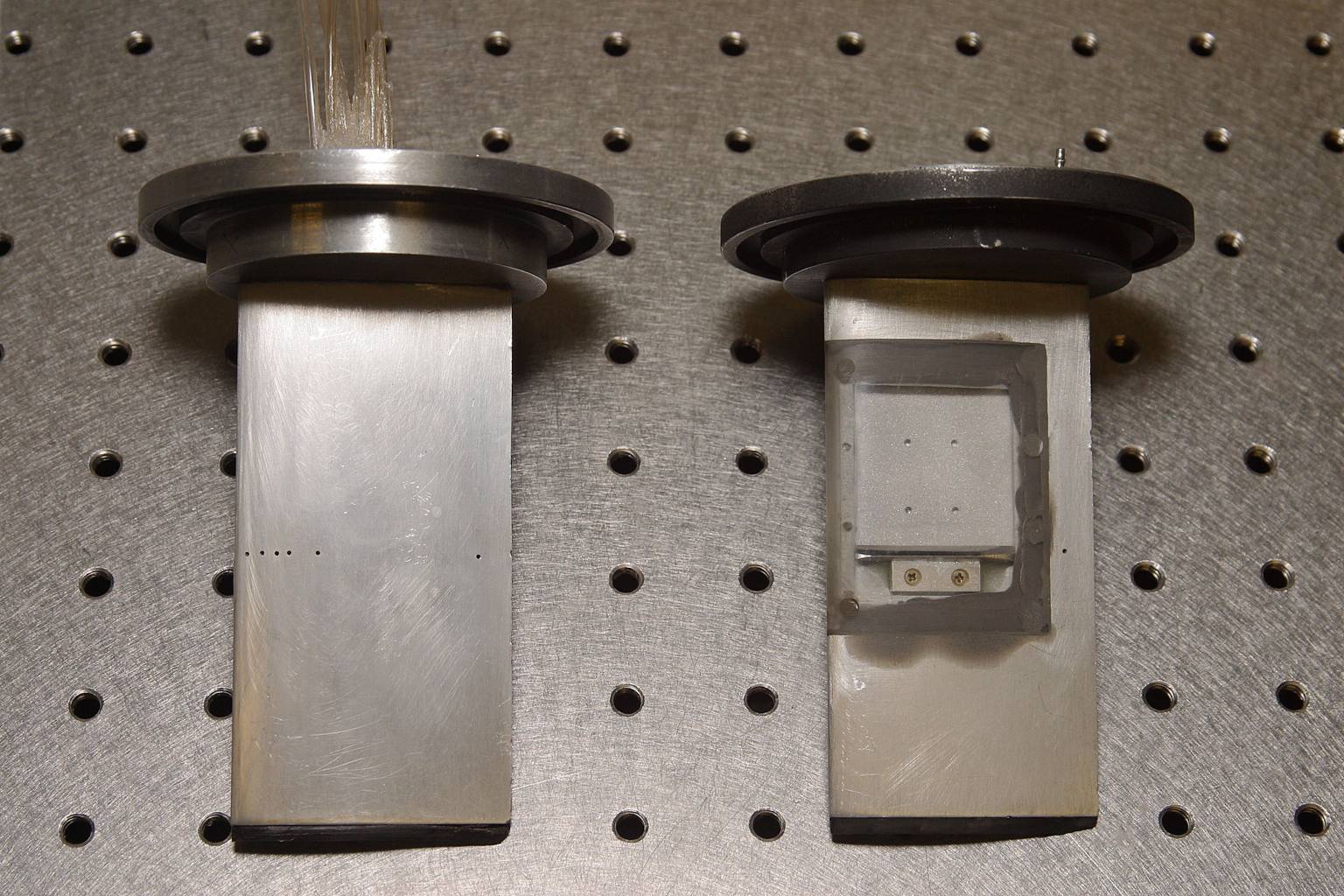
The two NACA0015 hydrofoils used in the tests. Left: Pressure tap foil with 7 taps on suction side and 2 on pressure side, all along centerline. Right: Laser sheet access foil, allowing UV laser sheet to be injected along the centerline from the surface of the hydrofoil.
A separate experimental setup has been constructed to study the spectroscopic properties of the fluoroketone used as a test medium.
References
- Gustavsson, J., Segal, C.,
Dorney, D., "Incipient Cavitation Studied under Strong
Thermodynamic Effect", AIAA Journal, 47 (3), pp.
710-716, 2009.
- Gustavsson, J. P. R., Denning, K., Segal, C., "Hydrofoil Cavitation under Strong Thermodynamic Effect", Journal of Fluids Engineering, 130 (9), 091303, 2008. (Abstract provided below.)
- Gustavsson, J. P. R., Denning, K., Segal, C.,
"Experimental study of Cryogenic Cavitation Using Fluoroketone", AIAA 2008-0576, 46th AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, Jan 7-10, 2008.
Links
To my CV
Back to main page
Back to main UF research page
Incipient cavitation was studied under simulated cryogenic conditions on a NACA0015
hydrofoil in a tunnel filled with the perfluorinated ketone 2-trifluoromethyl-1, 1,
1, 2, 4, 4, 5, 5, 5-nonafluoro-3-pentanone. Through pressure measurements on the
hydrofoil, laser-illuminated high-speed photography, and flash-illuminated photography,
the extent of cavitation and the characteristic frequencies of its oscillation were
studied under varying speeds in the range of 1.7-6.7 m/s and several angles of attack.
The results presented in this paper are limited to a 5.1-degree angle of attack. It was
found that the vapor formation was much stronger in fluoroketone than in cold-water tests
at similar cavitation numbers. The formed bubbles were significantly smaller and there
existed an extended speed range over which fluctuation amplitudes grew with no well-defined
frequency peaks, as was observed in water.
Cavitation was studied for a NACA0015 hydrofoil using a material that simulates cryogenic
behavior. Several angles of attack and flow speeds up to 8.6 m/s were tested. The material
used, 2-trifluoromethyl-1,1,1,2,4,4,5,5,5-nonafluoro-3-pentanone, hereafter referred to as
fluoroketone, exhibits a strong thermodynamic effect even under ambient conditions. Static
pressures were measured at seven chordwise locations along the centerline of the hydrofoil
suction side and on the test section wall immediately upstream of the hydrofoil. Frequency
analysis of the test section static pressure showed that the amplitude of the oscillations
increased as the tunnel speed increased. A gradual transition corresponding to the Type II-I
sheet cavitation transition observed in water was found to occur near sigma/2alpha=5 with Strouhal
numbers based on chord dropping from 0.5 to 0.1 as the cavitation number was reduced.
Flash-exposure high-speed imaging showed the cavity covering a larger portion of the chord
for a given cavitation number than in cold water. The bubbles appeared significantly smaller
in the current study and the pressure data showed increasing rather than constant static
pressure in the downstream direction in the cavitating region, in line with observations made
in literature for other geometries with fluids exhibiting strong thermodynamic effect.


