E-Museum: Machining the Body
- Young Machinist
Cell seeding and impregnation is an
indispensable step for research and development in tissue engineering,
regenerative medicine, biosensor/bioactuator, and other different
cell-associated biological applications. Homogeneous cells have been
conventionally seeded using soft lithography, dipping, microlitre syringe
dispatching, to name a few. As a result, seeded cell are usually uniform and
mostly in a two-dimensional (2D) form, which may not be sufficient for some
controlled gradient and/or three-dimensional (3D) cell applications.
As a promising
alternative, cell direct writing has emerged as a revolutionary advance in
tissue engineering with great potential for use in the manufacture of
arbitrary cell patterning as well as heterogeneous two or three-dimensional
(2D or 3D) living scaffolds [H1-H3]. Generally speaking, direct-writing
technologies include any techniques or processes capable of depositing,
dispensing, or processing different types of materials over various surfaces
to create structures with controlled architecture and composition in a
consequent manner based on any computer-aided design [H2, H4]. During a
typical direct-write approach, patterns or layered structures are built
directly without the use of masks, allowing rapid prototyping. Either the
substrate or the direct-write head or the both can be designed to be movable
with respect to each other in order to fabricate 2D or 3D patterns.
By utilizing fluid
jetting phenomena, maskless jet-based (mainly laser- or ink-based)
direct-write technologies as shown in Fig. H1, also known as jet-based
technologies, have been most pioneered to precisely position both nonviable
and viable biological patterns and constructs over different substrates [H1,
H5, H6]. For example, successful inkjet printing endeavors include E. coli
bacteria [H7] and viable mammalian cells [H8] deposition using a modified
thermal inkjet printer. Laser-based technologies mainly include the use of
laser light to form living cell clusters [H9], matrix-assisted pulsed-laser
evaporation direct-write (MAPLE DW) to deposit 2D [H10] and 3D [H11] mammalian
cellular structures, and laser-induced forward transfer [H12] to assist rat
Schwann and astroglial cell deposition. Recently, electrohydrodynamic jetting
(EHDJ) method has also been successfully demonstrated to print viable cells
[H13] and even zebrafish embryos [H14].
Laser-based approach mainly includes laser
guidance direct writing using laser optical force and laser-induced forward
transfer (LIFT) and its modified forms using laser pulse energy, and the
aforementioned MAPLE DW is a form of LIFT. Ink-based approach relies on the
deposition of cell culture-based inks to create structures layer-by-layer
using mechanical pressure and/or electric force, and it mainly includes inkjet
direct writing and EHDJ. Using a bottom-up approach, different direct-write
methods are envisioned to seed cells and biomaterials to resemble a
multiculture, multimaterial, heterogeneous structure, which could be an
enhanced environment for regenerative growth and other medical treatment
purposes such as organ printing for donor shortage. The following sections
introduce some common cell direct-write technologies from the manufacturing
process development point of view.
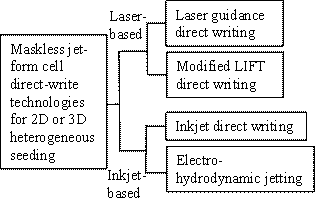
Fig. H1. Classification of jet-based cell
direct-write technologies
2.1 Laser-based
direct writing
2.1.1 Laser guidance direct writing
Laser guidance direct writing uses the optical force to direct write different
particles, including cells and other biomaterials. Optical force was first
demonstrated to levitate aerosol droplets and dielectric spheres in 1970 [H15].
Since that, various biological and inorganic materials have been successfully
manipulated optically in an aqueous suspension [H16-H18], and viable cells were
successfully patterned using a laser beam-based optical force [H9, H19].
The optical force utilized during cell direct writing comes from the scattering
of laser beam energetic photons off the surface of a microscopic particle. If
the refraction index of a particle is larger than that of the surrounding
medium, the particle experiences both a radial force pulling the particle
towards the center of laser beam and an axial force pushing the particle in the
propagation direction of the light [H1, H16]. The larger the difference of the
refraction indexes between the particle and the medium, the stronger the optical
force. Depending on whether a hollow optical fiber is used or not to minimize
the effect of the fluid motion from the suspension medium, laser guidance direct
writing is usually implemented in two different ways as shown in Fig. H2. For
most biomedical applications, since water absorbs less light energy from
near-infrared wavelength lasers, lasers with a near-infrared wavelength have
been favored to reduce possible the laser-induced thermal damage to cells [H18,
H20].
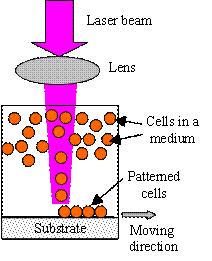
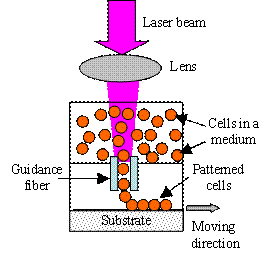
(a)
(b)
Fig. H2. Laser guidance direct writing system: (a)
simple setup and (b) system with a guidance fiber
During a laser guidance cell direct writing process, the cell droplet is
generally formed using sonic agitation or nebulization. Once the particle
droplets are formed, they are guided onto the substrate by either gas flow or
optical force momentum effects. The resultant droplet velocity is usually as low
as 10-5—10-4 m/s [H1].
2.1.2 Modified LIFT direct writing
Laser has also been
widely used to deposit different engineering materials using the high pressure
generated during the laser-matter interaction process. LIFT and MAPLE were
firstly demonstrated the capacity to deposit metals and dielectric materials
over various substrates. During the LIFT process, the coated transparent thin
film substrate (called the donor substrate) is paired with an uncoated substrate
(the acceptor substrate to which the pattern is to be deposited). A laser is
focused through the transparent donor substrate onto the thin film causing the
formation of vapor at the film-substrate interface and the ejection of a portion
of the film as a high speed jet. The ejected thin film material then strikes the
acceptor substrate, bonding with it. MAPLE is a variation of the conventional
pulsed-laser evaporation. It is a more gentle mechanism for transferring many
different compounds that include small and large molecular weight species such
as sugars and polymeric molecules, from the condensed phase into the vapor
phase. When a substrate is positioned in the path of the plume, a continuous
film is formed from the evaporated solute, while the lower molecular weight
solvent is rapidly removed by continuous evacuation. It is conducted in a
conventional vacuum chamber equipped with a window of high transmittance for the
wavelength of the pulsed laser to be used. The laser interaction primarily
occurs with the solvent, and the material of solute remains intact. The solute
concentration is intentionally kept low so that the incident laser energy is
mostly absorbed by the solvent molecules rather than by the solute molecules.
By combining the
beauties of LIFT and MAPLE, modified LIFT has been pioneered to transfer living
cells and other biomaterials [H1, H11, H21, H22]. As shown schematically in Fig.
H3, in MAPLE DW focused ultraviolet (UV) laser pulses are directed
perpendicularly through the backside of a UV laser transparent quartz support
that is coated with a solution of materials to be transferred. The laser pulses
are then absorbed by the matrix at the interface causing extremely localized
heating and evaporation of a small portion of the coating to form a small
vapor/plasma pocket. Finally, the sublimation releases the remaining beneath
coating as a droplet from the interface by ejecting it away from the quartz
support to a receiving substrate directly underneath (on the order of 100
um) [H23]. MAPLE DW is schematically similar to
laser-induced forward transfer (LIFT), but the matrix coating and thus the
novelty of the laser-material interaction are what makes MAPLE DW unique.
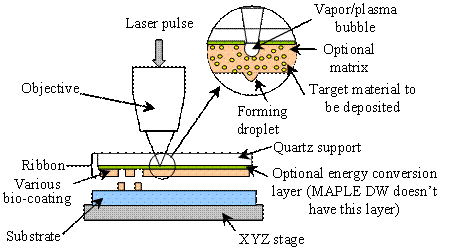
Fig. H3. MAPLE DW and other modified LIFT
schematic
2.2
Inkjet-based direct writing
2.2.1 Inkjet direct writing
Inkjetting technology recently has been modified to successfully print E.
coli bacteria [H7] and viable mammalian cells [H8] as a promising tissue
engineering technology. A typical inkjet system consists of a printhead and an
ink cartridge/reservoir, and the bio-ink is delivered through one of the two
main mechanisms: thermal and piezoelectric. Regardless of the working mechanism,
an inkjet direct-write process involves three stages: pressure generation, cell
droplet formation and ejection through an orifice, and cell droplet landing on a
receiving substrate. The cell droplet velocity in inkjet printing is usually
around 10 m/s [H1].
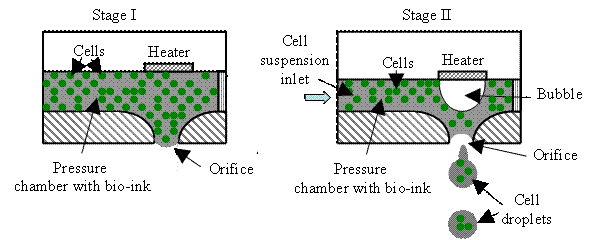
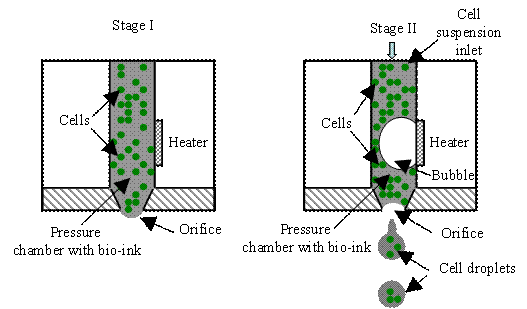
As shown in Fig. H4,
a thermal inkjet system can be implemented as roof-shooter or side-shooter using
the following mechanism. First, a drive circuitry is used to produce a heating
pulse that can raise the temperature of a heating element to several hundred
degrees. Then a bubble is formed near the element surface, producing a bubble
expansion-induced outwards pressure. This pressure forces a controlled amount
bio-ink, which is in the pressure chamber, out of the orifice as an ink jet.
Finally, the ejected bio-ink forms a cell droplet towards a receiving substrate.
The collapse of bubble pulls bio-ink from the cell suspension inlet into to fill
the pressure chamber, and the cell suspension inlet usually connects to a
bio-ink reservoir. Since most biological materials such as living cells,
proteins, peptides, nucleic acid, and various nucleotide bases are very
sensitive to heat, excessive heat should be controlled to avoid any possible
thermal damage to these biological materials.
(a)
(b)
Fig. H4. Two thermal inkjet systems: (a)
roof-shooter and (b) side-shooter
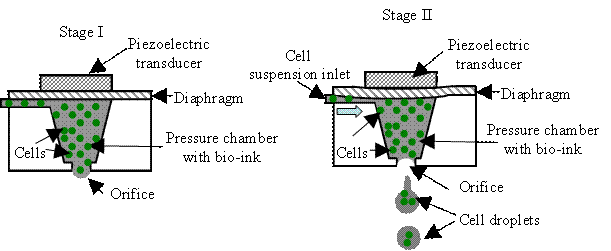
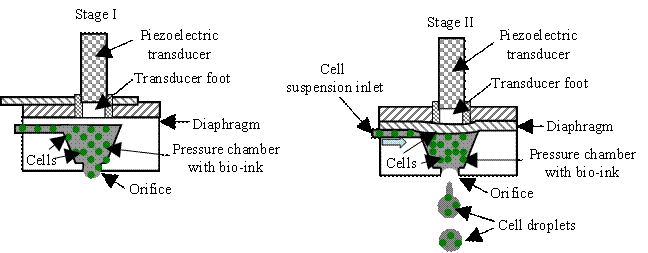
As shown in Fig. H4, a piezoelectric inkjet system can be implemented in bend or
push mode using the following mechanism. First, a controlled voltage pulse is
applied to a piezoelectric element, and the deformation of diaphragm shape
causes a contraction in the volume of the bio-ink pressure chamber, jetting a
controlled amount of fluid from an orifice. Then the ejected bio-ink forms cell
droplet and lands on a receiving substrate. The relaxation of the piezoelectric
element causes the pressure chamber to return to its original geometry,
refilling from a connected cell suspension inlet. While the process-induced
temperature rise is negligible in a piezoelectric inkjet system, cells are
exposed to deleterious electric fields [H26].
(a)
(b)
Fig. H5. Two piezoelectric inkjet
systems: (a) bend-mode and (b) push-mode
In inkjet-based cell direct writing, the thermal or piezoelectric printheads
and ink cartridges should be charged with cell culture. Besides,
biocompatibility requirement must be fulfilled before using an inkjet system to
direct write biomaterials. When heterogeneous patterns of cells are incorporated
into a tissue scaffold, another requirement is multifunctional direct writing,
which means that several types of cells and cultures can be arranged in order to
manufacture biological tissues [H26]. Some other inkjetting-evolved
biodispensing technologies [H2]
have also been successfully applied to deposit living cells in both continuous
extrusion and drop-on-demand droplet modes [H27] as shown in Fig. H6.
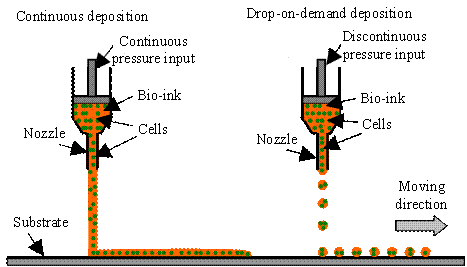
Fig. H6. Biodispensing deposition modes:
continuous extrusion and drop-on-demand droplet
During MAPLE DW, a matrix is selected as the laser energy absorbing material.
For some other applications, a sacrificial coating layer, which functions as the
laser energy absorbing vehicle is coated between the quartz support and the
biological coating to be transferred, forming a sandwich structure [H10-H12,
H24, H25] as illustrated in Fig. H3. While such a laser-assisted deposition
approach is named as absorbing film-assisted LIFT (AFA LIFT) and biological
laser printing (Bio LP) [H10-H12, H24, H25], it is usually classified as
modified LIFT [H1].
2.2.2 Electro-hydrodynamic jetting
Electro-hydrodynamic jetting (EHDJ) has emerged as a new endeavor in cell direct
writing. As shown in Fig. H7, during EHDJ the bio-ink is pumped through a
needle-like nozzle, which is connected to a high voltage power supply, using a
syringe pump. Jetting of the bio-ink is controlled by adjusting the strength of
high voltage-induced electric field which is on the order of 1 kV/mm. At the
nozzle outlet, the applied high voltage and Taylor instability make the ejected
bio-ink into droplets under the effects of surface tension and electric field
gradients.
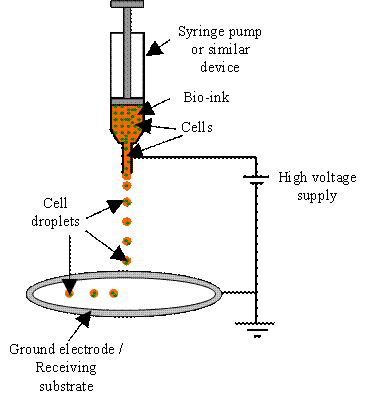
Fig. H7. Schematic of electro-hydrodynamic
jetting
The distance between the nozzle outlet and the target substrate is usually on
the order of 10 mm, which may result in some deleterious evaporation or cooling
effects to cells [H1]. The cell droplet velocity in EHDJ is about 0.1 m/s, which
is far lower than that in inkjetting. The high applied voltages and strong
electric field gradients during EHDJ can induce deleterious damage to cells in
the bio-ink; however, some successes have been achieved in direct writing of
various biological constructs including zebrafish embryos [H14].
Acknowledgement:
This work of this web site was partially
supported by the National Science Foundation (NSF), the National Textile Center
(NTC), and the South Carolina Space Grant Consortium (SCSGC).
[H1] Ringeisen, B. R., Othon, C. M., Barron, J. A.,
Young, D., and Spargo, B. J., 2006, "Jet-based methods to print living cells,"
Biotechnology Journal, Vol. 1, pp. 930-948.
[H2] Lewis, J. A., and Gratson, G. M., 2004,
"Direct
writing in three dimensions," Materials Today, Vol. 7(7/8), pp. 32- 39.
[H3] Barbulovic-Nad, I., Lucente, M., Sun, Y., Zhang, M.,
Wheeler, A. R., and Bussmann, M., 2006, "Bio-microarray fabrication techniques
- a
review," Critical Reviews in Biotechnology, Vol.
26, pp. 237-259.
[H4] Piqué, A., and Chrisey,
D. B., 2001, Direct-write
technologies for rapid prototyping applications: sensors, electronics, and
integrated power sources, Academic Press, New York, 1st edition.
[H5] Langer, R., and Vacanti, J.P., 1993,
"Tissue
engineering," Science, Vol. 260(5110), pp. 920 - 926.
[H6] Saltzman,
W. M., 2004, Tissue engineering:
engineering principles for the design of replacement organs and tissues,
Oxford University Press, New York, 1st edition.
[H7] Xu, T., Petridou, S., Lee, E. H., Roth, E. A.,
Vyavahare, N. R., Hickman, J. J., and Boland, T., 2004, "Construction of
high-density bacterial colony arrays and patterns by the ink-jet method,"
Biotechnology Bioengineering, Vol. 85(1), pp. 29-33.
[H8] Xu, T., Jin, Y., Gregory, C., Hickman, J. J., and
Boland, T., 2005, "Inkjet printing of viable mammalian cells,"
Biomaterials,
Vol. 26(1), pp. 93-99.
[H9] Odde, D. J., and Renn, M. J., 2000,
"Laser-guided
direct writing of living cells," Biotechnology Bioengineering, Vol. 67, pp.
312-318.
[H10] Ringeisen, B.R., Kim, H., Barron, J.A., Krizman, D.B.,
Chrisey, D.B., Jackman, S., Auyeung, R.Y., and Spargo, B.J., 2004,
"Laser
printing of pluripotent embryonal carcinoma cells," Tissue Engineering, Vol. 10,
pp. 483-491.
[H11] Barron, J. A., Ringeisen, B. R., Kim, H., Spargo, B.
J., and Chrisey, D. B., 2004, "Application of laser printing to mammalian
cells," Thin Solid Films, Vol. 453-454, pp. 383-387.
[H12] Hopp, B., Smausz, T., Kresz, N., Barna, N., Bor,
Z., Kolozsvari, L., Chrisey, D.B., Szabo, A., and Nogradi, A., 2005,
"Survival
and proliferative ability of various living cell types after laser-induced
forward transfer," Tissue Engineering, Vol. 11(11/12), pp. 1817-1823.
[H13] Jayasinghe, S.N., Qureshi, A.N., Eagles, P.A.M.,
2006, "Electric field driven jetting: an emerging approach for processing living
cells," Biotechnology Journal, Vol. 1, pp. 86-94.
[H14] Clarke, J. D. W., and Jayasinghe, S.N., 2008, "Bio-electrosprayed
multicellular zebrafish embryos are viable and develop normally," Biomedical
Matererials: Materials for Tissue Engineering & Regenerative Medicine, Vol. 3,
pp. 011001.
[H15] Ashkin, A., 1970,"Acceleration and trapping of
particles by radiation pressure," Physical Review Letter, Vol. 24, pp. 156-159.
[H16] Ashkin, A.
1980," Applications of laser radiation pressure," Science, Vol. 210(4474),
pp.1081-1088.
[H17] Ashkin, A., and Dziedzic, J. M., 1987, "Optical
trapping and manipulation of viruses and bacteria," Science, Vol. 235(4795), pp.
1517-1520.
[H18] Liang, H., Vu, K. T., Krishnan, P., Trang, T. C.,
Shin, D., Kimel, S., and Berns, M. W., 1996, "Wavelength dependence of cell
cloning efficiency after optical trapping," Biophysical Journal, Vol. 70, pp.
1529-1533.
[H19] Odde, D. J., and Renn, M. J., 1999,
"Laser-guided
direct writing for applications in biotechnology," Trends Biotechnology, Vol.
17, pp. 385-389.
[H20] Konig, K., Tadir, Y., Patrizio, P., Berns, M. W.,
Tromberg, B. J., 1996, "Effects of ultraviolet exposure and near infrared laser
tweezers on human spermatozoa," Human Reproduction, Vol. 11(10), pp. 2162-2164.
[H21] Piqué, A., Chrisey, D. B., Auyeung, R. C. Y.,
Fitz-Gerald, J., Wu, H. D., McGill, R. A., Lakeou, S., Wu, P. K., Nguyen, V.,
and Duignan, M., 1999, "A novel laser transfer process for direct writing of
electronic and sensor materials," Applied Physics A: Materials Science &
Processing, Vol. 69(7), pp. 279-284.
[H22] Ringeisen, B.R.,
Chrisey, D.B., Pique, A., Young, D., Modi, R., Bucaro, M., Jones-Meehan, J., and
Spargo, B.J., 2002, "Generation of mesoscopic patterns of viable Escherichia
coli by ambient laser transfer," Biomaterials, Vol. 23, pp. 161-166.
[H23] Lin, Y., Huang, Y., and Chrisey, D.B., 2009, "Droplet
formation in matrix-assisted pulsed-laser evaporation direct writing of
glycerol-water solution," Journal of Applied Physics, Vol. 105, pp.
093111-1-6.
[H24] Barron, J. A., Wu, P., Ladouceur, H. D., and
Ringeisen, B. R., 2004, "Biological laser printing: a novel technique for
creating heterogeneous 3-dimensional cell patterns," Biomedical Microdevices,
Vol. 6, 139-147.
[H25] Chen, C. Y., Barron, J. A., and Ringeisen, B. R.,
2006, "Cell patterning without chemical surface modification: cell-cell
interactions between bovine aortic endothelial cells (BAEC) on a homogeneous
cell-adherent hydrogel," Applied Surface Science, Vol. 252(24), pp. 8641-8645.
[H26] Nakamura, M., Kobayashi, A., Takagi, F., Watanabe,
A., Hiruma, Y., Ohuchi, K., Iwasaki, Y., Horie, M., Morita, I., and Takatani,
S., 2005, "Biocompatible inkjet printing technique for designed seeding of
individual living cells," Tissue Engineering, Vol. 11(11-12), pp. 1658-1666.
[H27] Khalil, S., Nam, J., and Sun, W., 2005,
"Multi-nozzle
deposition for construction of 3D biopolymer tissue scaffolds,"
Rapid
Prototyping Journal, Vol. 11(1), pp. 9-17.









