
I. INTRODUCTION - Lipids are characterized by being insoluble in water and extracted by nonpolar solvents, such as chloroform or ether. The major types of lipids are grouped according to their chemical structure. There is no functional group or groups that defines this large family of molecules.
II. FATTY ACIDS - the fatty acids are monocarboxylic acids containing about 6 to 26 carbons in the form of hydrocarbons and serve as the building blocks of many lipids.
- The number of carbons is always an even number, and their biosynthesis occurs two carbons at a time.
- The fatty acids differ in the number of carbons and the presence, number and position of double bonds in the hydrocarbon chain.
- The unsaturated forms are more abundant than the saturated forms and the double bond usually occurs between C-9 and C-10.
- If other double bonds are present they occur toward the methyl terminal, and are never conjugated.
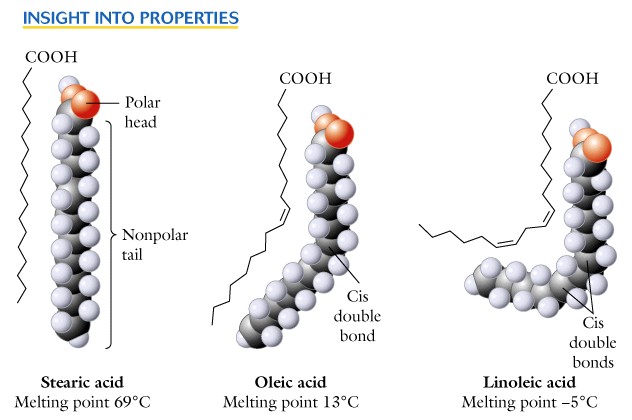
As you recall the carbon-to-carbon double bond is not free to rotate and the cis/trans configuration is possible. The unsaturated fatty acids are usually in the cis-configuration producing a rigid bend at that point.
The saturated C12 to C24 are solids with a waxy consistency, whereas the unsaturated ones are oily liquids at ambient temperatures.
Fatty acids are insoluble in water, but the polar carboxyl group will associate with water and the hydrocarbon part will not, i.e. there is a hydrophilic and a hydrophobic part of each molecule.
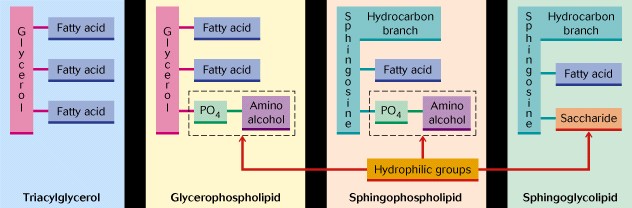
III. TRIACYLGLYCEROLS - these are fatty acid esters of the alcohol glycerol, and are often called fats, neutral fats, or triglycerides. They are formed by the reaction between each of the three hydroxyl groups of glycerol and three fatty acid molecules via the removal of water and the formation of the ester linkage. The oxygen of the ester linkage comes from glycerol. If the fatty acids at C1 and C3 are different, then C2 is chiral and D- and L-forms are possible. The natural ones show the L-form.
Triacylglycerols are excellent energy storage molecules, and are usually stored as pure droplets in special cells called adipocytes, a specialized type of connective tissue of animals.
The energy content for a typical triacylglycerol is 9.3, typical carbohydrate 3.79, and a typical protein 3.12 kcal/g.
Recall the examples of the Ruby-throated humming bird and the sperm whale given in lecture.
Droplets of triacylglycerols in small migrating birds may represent nearly 50% of the body weight.
IV. WAXES - waxes are esters of long-chained saturated and unsaturated fatty acids (14-36 carbons) with long-chained alcohols (16-22 carbons).
- Skin, hair, wool, and fur secrete waxes.
- Birds, especially waterfowl, secrete waxes from their preen glands to make feathers water-repellent.
- Leaves of plants have a waxy coating.
- Plankton form waxes as their chief storage molecules, and marine species feeding on plankton use waxes as the major food in oceanic food chains.
V. PHOSPHOGLYCERIDES or PHOSPHOLIPIDS - phospholipids are major components of membranes and they differ from triacylglycerols in having one or more polar head groups. The most abundant ones use a phosphoric acid molecule in place of one of the three fatty acid molecules. Thus on carbon #1 and #2 ester linkages between two fatty acids are formed and on carbon #3 a phosphoester linkage with phosphoric acid is formed. A second alcohol is then usually added to the phosphoric group with the same linkage. The second alcohol gives the name to the molecule, and the two most common ones are ethanolamine and choline, two others are serine and inositol. All phospholipids have a negative charge on the phosphoric acid group at pH 7. The head alcohol may also contribute one or more charges, i.e. ethanolamine is +, choline is + and serine is both + and - . Thus they are termed amphipathic since they have a polar hydrophilic head and nonpolar hydrophobic tails.
Recall the symbol use to show the structure of the bilipid layer forming all membranes.
VI. SPHINGOLIPIDS- are a second large class of membrane lipids. They contain one molecule of a fatty acid, one molecule of a long-chained amino alcohol called sphingosine or a derivative of sphingosine, and a polar alcohol. Sphingosine contains 18 carbons, one double bond, an alcohol functional group, and an amino functional group. The alcohol group forms an ester bond with phosphocholine, and the amino group forms an amide linkage with a fatty acid. These molecules are abundant in the myelin sheath and are present in most other membranes.
VII. STEROIDS or STEROLS - All lipids covered to this point are saponifiable i.e. they are hydrolyzed by heating with alkali to yield soaps of their fatty acid components.
The sterols and the terpenes are nonsaponifiable. As we noted in lecture the steroids contain four fused rings in a distinctive structure and we have learned this basic structure.
- Cholesterol is a good example of the various modifications which provide the distinctive functional ability of steroids. Carbon #3 contains a hydroxyl functional group which provides the polar head group of the molecule, there is a double bond between carbons #5 and #6, and an eight carbon alkane chain at carbon #17. Finally, there are methyl groups at carbon #10 and #13.
The figure on the left is the steroid nucleus containing the four fused
rings (A,B,C,D).
Examine the structure of other steroids in the textbook and note the favored sites for inducing modifications in the molecule.
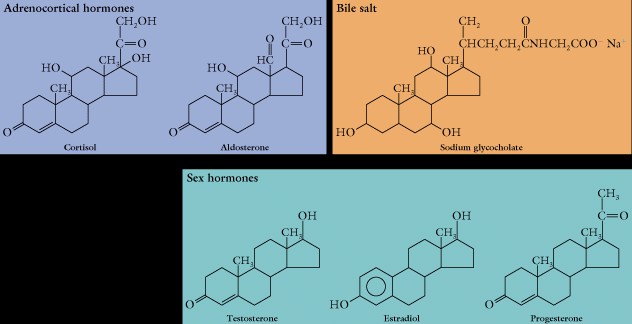
Cholesterol is the parent compound for the various steroids made in animals, in plants the most common is stigmasterol, which differs only in the presence of a double bond at carbon #22 an #23.
The androgens and estrogens are called the sex hormones and have significant regulatory effects in animals.
Note how cholesterol integrates itself into the bilipid layer.
Recall the basic structure of terpenes and especially isoprene (2-methyl-1,3-butadiene), the building block of vitamin A, beta-carotene, and the visual pigments.
VIII. LIPOPROTEINS - blend the properties of lipids and proteins and are common in blood. They contain from 50 to 90% lipid with the balance being protein, but contain no covalent linkages between the lipid and the protein. The levels of these molecules in blood plasma is an important factor in arteriosclerosis (clogging of blood vessels).