
![]() This is a summary
of Isomer Nomenclature, as applied to the various organic families.
This is a summary
of Isomer Nomenclature, as applied to the various organic families.
I. Constitutional - isomers are characterized by having different connections among the atoms and the free rotation of single covalent bonds. The major examples are:
(1) structural - review the several types such as skeletal, functional group, and positional isomers;
(2) conformational - review the concept of the free rotation of single bonds and how this leads to a number of possible shapes of hydrocarbons such as, ethane and cycloalkanes.
Note that there is a preferred shape for each of these molecules.
![]()
II.Configurational - isomers are characterized by having a different geometry, and thus cannot be interconverted without bond breakage. There are a number of examples and some major ones include:
(1) geometric isomers based on the presence of the carbon to carbon double bond, and termed cis/trans. When each carbon atom making up the double bond has two different functional groups attached, these functional groups may be on the same or different sides of the double bond. When on the same side this is called cis and when on different sides it is called trans. Review several examples.
(2) enantiomers - sometimes termed optical or stereo-isomers, occur in pairs as D or (+) and L or (-), + and - refer to direction of rotation of plane polarized light, D and L refer to the specific arrangement using glyceraldehyde as the model molecule, these two forms are not superimposable on their mirror image, require the presence of one or more chiral carbon atoms. Recall that a chiral carbon atom has four different substituent groups bonded to it.
Study the structural formula for D-(1) and L-(2) glyceraldehyde and be sure that you learn these two molecules, since they are the basis for all D- and L-forms.

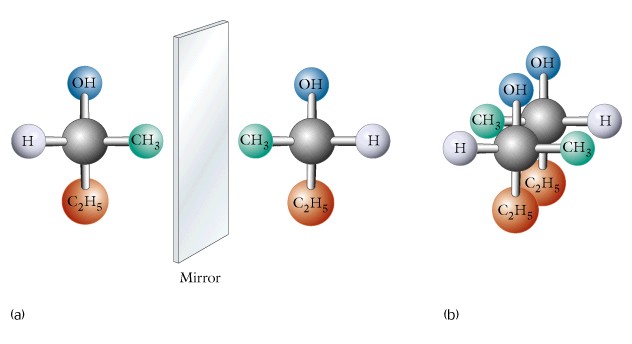
(3) meso compounds - have 2 or > chiral carbons, but differ from enantiomers in that they show mirror images and a plane of symmetry. An example is meso-tartaric acid, which contains two chiral carbons each with a hydrogen, hydroxyl, and carboxyl functional group. Write out the structures of these molecules and note that there is a plane of symmetry. Also note that meso compounds are different from enantiomers, and extend the basic concept of D- and L-forms.
(4) diastereomers - have non-mirror images, contain 2 or > chiral carbons, and they have a relationship somewhat like that of hand and foot, rather than right and left hands of enantiomers. Note that these molecules also extend the concept of D- and L-forms.
(5) anomers - are based on ring closure between a carbonyl functional group and an alcohol functional group contained within the same molecule. This is termed hemiacetal or hemiketal formation and is a common reaction between an aldehyde or ketone and an alcohol. This ring closure, between forms a new chiral carbon and the two resulting forms are termed alpha and beta.
By definition alpha shows the hydroxyl on C1 down or trans to C6 and beta shows the hydroxyl up or cis to the same carbon. Note this is an intramolecular reaction between the two functional groups, alcohol and aldehyde, to produce an ether of the heterocyclic type i.e. a pyran or furan ring.
Write out the structure of beta-D-glucose, and be sure that you can show the formation of these two anomers. Note that these molecules also extend the concept of D- and L-forms.
![]()
![]() Use this outline
as a guide to organizing your study of the importance of the shape of
molecules. These concepts and terms will be applied to many biological
molecules, and it is of interest to note that:
Use this outline
as a guide to organizing your study of the importance of the shape of
molecules. These concepts and terms will be applied to many biological
molecules, and it is of interest to note that:
- all cells use D-sugars rather than L-sugars or a mixtureof D- and L-forms;
- all cells use L-amino acids rather than D-amino acids or a mixture;
- all cells use phosphatides (among the lipids) in the L-form rather than the D-form or a mixture.
What is the advantage, to cells, of using only one form of a molecule that has the natural ability to exist in two forms?
Recall the term racemic mixture, which is defined as containing equal amounts of an enantiomeric pair, indicates that from a chemical point of view both forms are equally possible.