I. Introduction - Carbohydrates are our first group of biomolecules and we want to review the concept that most biomolecules are polyfunctional and that most contain one or more chiral or asymmetric carbon atoms.
Carbohydrates are either aldehydes or ketones and polyhydroxy alcohols with one or more chiral carbon atoms, and a basic alkane structure.
Remember that the reference compound for D- and L-forms is glyceraldehyde and in giving the structure of the molecule you place the most oxidized carbon atom at the top and bonded to the chiral carbon atom. The D-form is defined as having the hydroxyl group written to the right of the chiral carbon and the hydrogen atom written to the left. This arrangement is reversed in the L-form. All sugars with one or more chiral carbons having a configuration of D- or L-form are related to glyceraldehyde and its two defined forms.
Review Appendix E in your text and note especially the Sequence Rules for the Order of Priority of the various functional groups in the R/S System of nomenclature.
Some interesting facts about carbohydrates are the following:
(1) A typical human diet consists of about 60% carbohydrate, on a dry weight basis, mainly sugars and starch.
(2) The carbohydrate cellulose is the most abundant organic chemical on earth.
(3) The process of photosynthesis produces some 400 billion pounds of carbohydrate/year.
(4) The major blood group types are determined by sequences and branches of carbohydrates attached to the surface of the cells. Add several facts that you know about carbohydrates.
![]()
II. Classification of Carbohydrates - As polyhydroxy aldehydes or ketones or substances that yield them on hydrolysis, carbohydrates are hydrates of carbon and thus contain (CH2O)n. Some may also contain N, P, or S.
(1) Monosaccharides - contain single unit sugars (for example glucose) that are colorless, crystalline solids which are freely soluble in water and insoluble in nonpolar solvents.
There are two groups based on the structure of the carbonyl functional group as an aldehyde or as a ketone.
- The name ending for sugars is -ose and thus we have aldoses and ketoses.
The aldoses have the carbonyl in the aldehyde configuration at the end of the carbon chain, examples are glyceraldehyde, glucose, and ribose.
The ketoses have the carbonyl group at any position other than at the end of the carbon chain, examples are dihydroxyacetone (the ketone equivalent of glyceraldehyde), and fructose.
- The number of carbon atoms in the molecule also is the basis of naming, i.e. triose, pentose, and hexose.
Remember the names of the Alkanes? All monosaccharides, except dihydroxyacetone, contain one or more chiral carbon atoms and thus occur in the isomeric form of D- or L-derivatives. In those cases where more than one chiral carbon atoms occur in the molecule, the assignment of D- or L-form refers to the configuration of the chiral carbon the most distant from the carbonyl carbon.
- The naming of ketoses follows that of aldoses and is done by inserting -ul- before the ending -ose into the name of the corresponding aldose, for example the aldopentose D-ribose becomes the ketopentose D-ribulose.
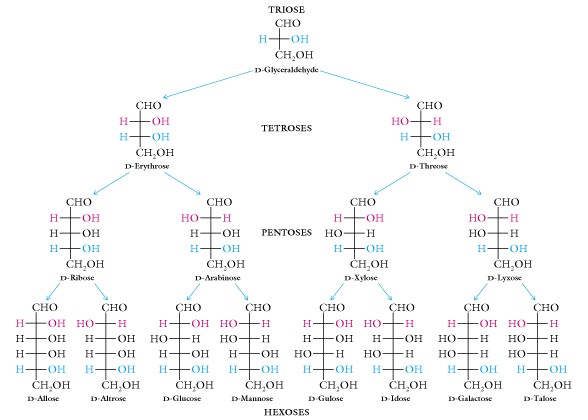
- The Aldose family:
- The Ketose family:
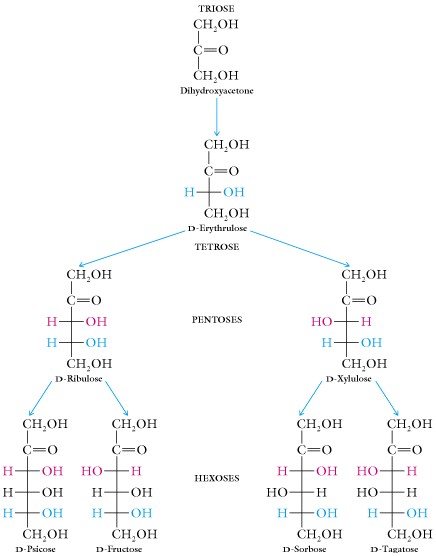
When two sugars differ only in configuration around one specific carbon atom they are termed epimers. An example is D-glucose and D-mannose , they are epimers with respect to carbon atom #2; another example is D-glucose and D-galactose, they are epimers of carbon #4.
Review the ring formation of alpha-D- and beta-D-glucose, and note that the naming refers to the ether ring pyran, i.e. beta-D-glucopyranose.
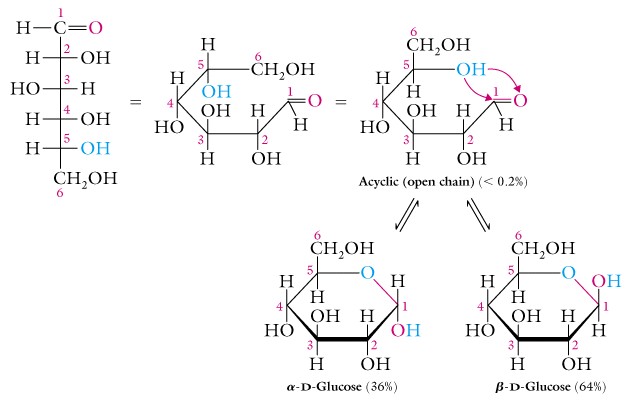
Most solutions of a monosaccharide actually consist of a complex mixture of alpha- and beta- forms of both pyran and furan configurations, but this is a complication that we will not spend much time on.
![]()
(2) Oligosaccharides - short chains of a few monosaccharides joined together as a disaccharide or a trisaccharide, etc.
The chemical bond or linkage between two monosaccharides to form a disaccharide is called a glycosidic bond .
This bond is formed by removing the elements of water, a hydroxyl from one sigar and a hydrogen atom from the hydroxyl of the other sugar, forming a C-O-C linkage between the two sugars.
An example is maltose or alpha-D-glucopyranosyl-(1->4)-beta-D-glucopyranose.
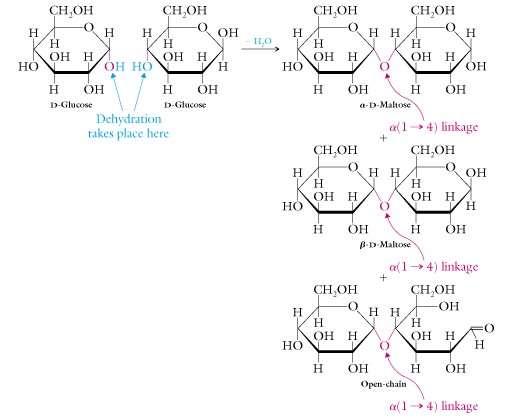
Write out the structures of these two glucose molecules and make the linkage between them. Is the overall molecule alpha or beta? Is the linkage between them alpha or beta?
Another important disaccharide is sucrose, a glucose and fructose dimer with an alpha-(1->2)-linkage, look in the textbook for the structure of this molecule.
![]()
(3) Polysaccharides - long chains having hundreds or thousands of monosaccharide units linked together with a glycosidic bond.
- Some of these polymers are linear (cellulose),
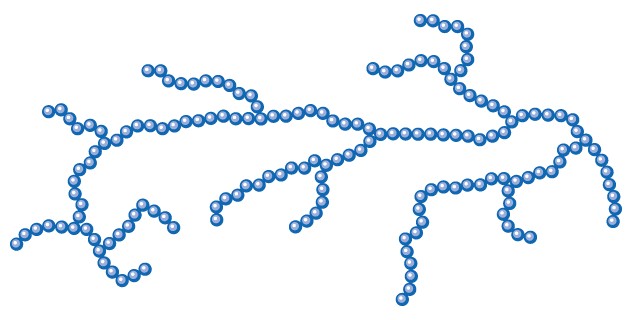
- others are branched (starch and glycogen),
- some have a single type of monomeric units (homopolymer),
- other more than on type of simple sugar (heteropolymer).
These are another example of the concept of monomer->polymer.
Review the structure of starch, and think ahead to the enzyme hydrolysis of this polymer.

Representation of the branching pattern. Each circle represents a glucose residue.
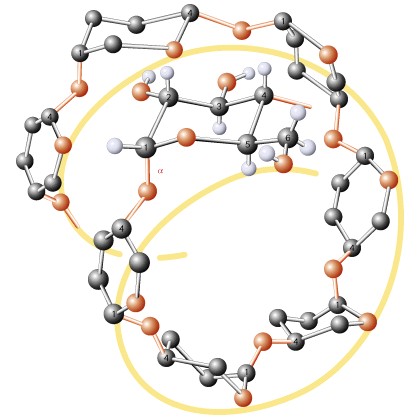
Helical conformation of amylose stabalized by intramoloecular hydrogen bonding:
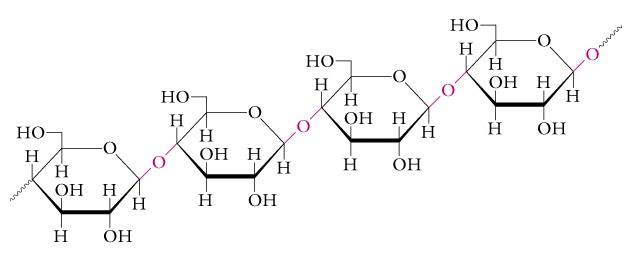
Cellulose a beta-1,4 glucose polymer:
Another polymer, chitin, uses an amino sugar as the monomeric unit, N-acetyl-D-glucosamine, linked via beta-(1->4)-bonds.
![]()
III. Chemical Reactions of Carbohydrates - Since carbohydrates contain a number of different functional groups, a number of chemical reactions are found. Some of the more common reactions are:
(1) formation of glycosides - the cyclic or pyran/furan form of monosaccharides is an acetal or ketal containing a hydroxyl group on carbon #1. The addition of a simple alcohol, methanol, to this position, results in the production of water and the methyl-glucoside of the monosaccharide.
(2) nucleotides and nucleic acids - Nucleotides are the monomeric units which produce the polymers termed nucleic acids (DNA & RNA). A nucleotide consists of a base, such as thymine, the sugar ribose or beta-2-deoxy-D-ribose, and a phosphoric acid residue. Thus the nucleotide is a monophosphate ester of the nucleoside, which consists of sugar plus base. We will spend more time on this when we reach the nucleic acids.
(3) cyanogenic glycosides - These are interesting molecules are formed by certain plants and contain a sugar plus the cyanide group. When the glycosidic bond is broken HCN is released . Thus providing protection to a plant from feeding insects.
(4) phosphate esters - In these molecules the hydroxyl group of the sugar forms an ester linkage with phosphoric acid. Remember that this acid has three hydroxyl groups, and mono-, di-, and tri-esters are possible. These esters of phosphoric acid are especially important in the biological world, in the structure of nucleic acids, and as the basis of energy metabolism. Both of these topics to be covered in detail at a later date.
(5) Reduction Reactions - The carbonyl group in glucose can be reduced to the alcohol with a reducing agent like sodium borohydride (NaBH4). The product is called glucitol.
(6) Oxidation of Sugars - Sugars are classified as reducing or non-reducing sugars, depending on their action with solutions like Benedict's or Tollen's reagent. The first reagent contains copper and the second silver. Sugars are oxidized to the corresponding acid at the carbonyl group, and the oxidizing agent becomes reduced. The measurement of the amount of an oxidizing agent that is reduced by a solution of sugar allows the amount of sugar present to be measured. The above solutions change color and are used to determine the amount of glucose in blood or other body fluids. Review the electronic structure of Cu and Ag to see the basis of the reaction.
(7) Recall our discussion of sweetness and the role of high-fructose corn syrup.