
I. Amines - Amines may be considered to be derivatives of ammonia (NH3) where one, two or all three of the hydrogen atoms are replaced by carbon functional group or groups yielding primary, secondary, and tertiary amines respectively. The IUPAC nomenclature drops the (-e) of the alkane name and adds (-amine), thus a primary amine is methanamine or CH3NH2 and the common name is methyl-amine.
Amines act as weak bases in water, and nitrogen uses three SP3 hybrid orbitals to form sigma bonds with three other atoms. The non-bonding lone pair of electrons occupy the fourth corner of a tetrahedron with bond angles of 109 degrees.
Amines are polar since there is a large enough difference in the value of the electronegativity between hydrogen and nitrogen to provide a delta charge on each atom. Thus the amine functional group can serve as a hydrogen donor in hydrogen bond formation.

Quaternary ammonium ion salts have four groups on the nitrogen and thus carry a positive charge.
The amino functional group (-NH2) occurs in amino acids, the monomeric units of the polymers termed proteins or polypeptides.
II. Heterocyclic aromatic amines - Heterocyclic aromatic amines are the abundant amines found in biological molecules.
The term heterocyclic means that one or more nitrogen atoms are present in a ring structure with several carbon atoms.

A good example is pyrrole, a five-membered heterocyclic amine consisting of four carbon atoms and one nitrogen atom. There are two double bonds linking each pair of carbon atoms. Pyrrole is both an amine and a conjugated diene becaue of the two double bonds, but its chemistry is not consistent with either of these functional groups. Unlike most amines, pyrrole is not basic and unlike most conjugated dienes it does not undergo electrophilic addition reactions. What is the explanation for these observations? Pyrrole is aromatic like benzene, and even though it has a five-membered ring it has six pi electrons pairs in a cyclic conjugated pi-orbital system. Each of the four carbon atoms contributes one pi electron, and the SP2 hybridized nitrogen atom contributes two more, its lone pair of unshared electrons in the P orbital. The six pi electrons occupy P-orbitals with lobes above and below the plane of the flat ring.
Be sure that you can compare this structure with that of benzene. Like benzene, pyrrole undergoes substitution of a ring hydrogen atom on reactions with electrophiles. Substitution normally occurs at the position next to nitrogen. Substituted pyrrole rings form the building blocks from which many plant and animal pigments are constructed. Among these is heme, an iron-containing tetrapyrrole found in hemoglobin.
Another example is pyridine, a nitrogen-containing heterocyclic analogue of benzene. Like benzene, pyridine is a flat molecule with bond angles of 120 degrees and with bond lengths of 1.39 units, a value which is intermediate between single and double bonds. Pyridine is aromatic with six pi electrons in a cyclic, conjugated pi-orbital system. The SP2 nitrogen atom and the five carbon atoms contribute one pi electron each to the orbital system. Unlike pyrrole, however, the lone pair of electrons on the pyridine nitrogen atom is not part of the pi orbital system, but occupies an SP2 orbital in the plane of the ring. Substituted pyridines occur in the B6-vitamins pyridoxal and pyridoxine, and these vitamins play important biological roles.
Another heterocyclic form is pyrimidine, containing two nitrogen atoms and four carbon atoms in a six member ring.
Several examples are cytosine, uracil, and thymine which occur in nucleic
acids.
The fused-ring aromatic heterocycles that contain both a benzene ring and a heterocyclic aromatic ring occur widely in biological molecules. Thus quinine, a quinoline derivative found in the bark of the tropical tree cinchona, is an important antimalarial drug. Lysergic acid, an indole derivative found in the ergot fungus that grows on grain, is the parent from which LSD is derived.
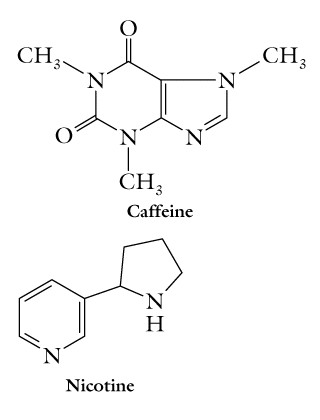
Another series of fused-ring forms contains two nitrogen atoms in each of the two fused rings, and include the purines adenine and guanine also found in nucleic acids.
III. Amides - Amides are amino derivatives of carboxylic acids, where the amino functional group replaces the hydroxyl group of the carboxyl functional group. The IUPAC system of nomenclature drops the (-oic acid) of the carboxylic acid and adds (-amide) in its place. An important use of this linkage is found in proteins where the amino functional group of one amino acid combines with the carboxyl functional group of a second amino acid to produce water and a linkage between the carbonyl group and the amino group. This linkage has special properties and is termed a peptide bond, and will attract our attention when we study amino acids and proteins.
IV. Imines - Imines are often termed Schiff bases and contain a carbon to nitrogen double bond. Ammonia and amines react with the carbonyl of aldehydes and ketones in the presence of acid to produce imines, where the carbon of the carbonyl group is now double-bonded to the nitrogen atom contained in ammonia or the amine.