
ALKANES & CYCLOALKANES
I. System of nomenclature for organic compounds (IUPAC) - The some 9 million organic compounds are classified into families according to their structural features and similar chemical reactivity. The chemistry of each organic molecule is determined by the functional group or groups that it contains.
- Functional groups are an atom or group of atoms that have a characteristic chemical behavior.
- The system of nomenclature for organic compounds is called IUPAC or the International Union of Pure and Applied Chemistry.
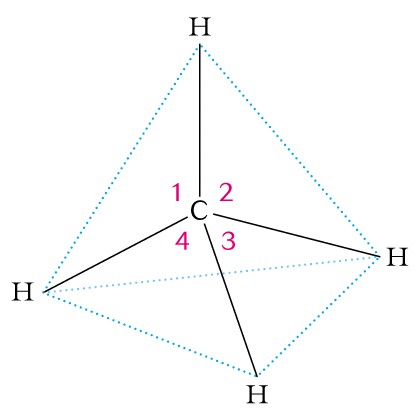
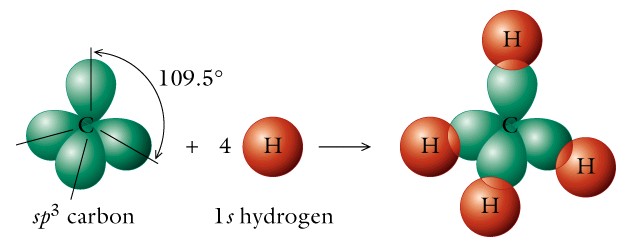
II. Hydrocarbons - The alkanes are saturated hydrocarbons and have the basic structure of CnH2n+2 and contain only single covalent bonds. The carbon atom is SP3 hybridized and has four equivalent valance electrons.
Review the hybridization of orbitals that was given in Lecture Notes l, and be sure that you understand this concept.
Alkanes may be straight-chained or branched-chain and thus may exist as structural or constitutional isomers. Such isomers have the same chemical formula, but have different chemical arrangements or structures and properties.
An alkyl functional group is formed when a hydrogen atom is removed from an alkane. For example, if a hydrogen is removed from methane (CH4), the functional group formed is called a methyl group (CH3-).
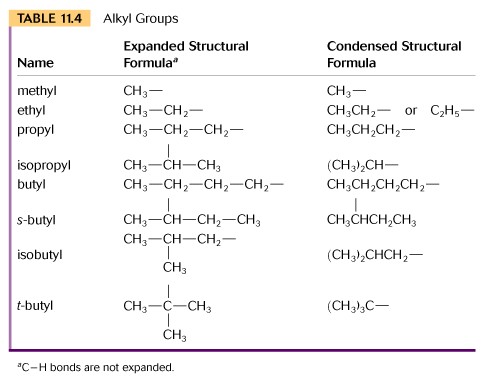
A good discussion of the rules of nomenclature for Alkanes is found Chapter 3 of your text. Please review this section now.
You are required to memorize the names of the first ten alkanes, since these are the parent compounds for all other organic families.
Note that there are generally three parts to a name:
- the prefix;
- the infix or parent compound; and
- the suffix or family. The family name ending for alkanes is -ane and the alkane that contains six carbon atoms would be called hexane.
III. Chemical reactions of alkanes - The chemical reactions shown by alkanes is suggested by one of their alternative names, the paraffins, from the Latin word meaning slight affinity. Thus they show little chemistry, undergoing combustion with molecular oxygen (O2) to yield carbon dioxide (CO2) and water (H2O) and energy in the form of heat.
You may recall that natural gas is composed of methane and has a relatively high energy content. The other main reaction is halogenation or the addition of a halogen to the molecule. These reactions require harsh conditions of temperature and pressure.
The major point to remember is that alkanes are highly reduced or energy-rich forms of carbon, and when you see a methyl group or some other alkane functional group in a molecule, it is likely to be stable and energy-rich.
IV. Shape of alkane molecules - One of the main points that we will make throughout this course is that the shape of molecules is important and is related to their function, i.e. structure/function relationships are the key to understanding biological molecules.
Alkanes have a conformation based on the specific arrangement of their atoms caused by the free rotation around single covalent chemical bonds. Thus a two-carbon alkane like ethane can exist in a large number of forms, although it has been found that certain forms are more stable and thus are favored. The favored form for ethane is termed staggered, and the reason seems to be that in this shape the bonding electron pairs are farthest from one another, thus minimizing the repulsion between these negatively charged particles. This is best seen in 3-D renditions.
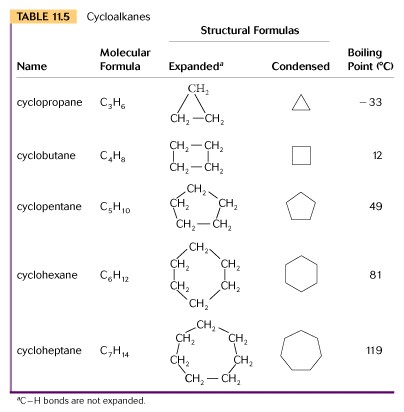
V. Cycloalkanes - Cycloalkanes are formed when 3 to 7 carbon atoms occur in a ring structure, rather than a linear one. In a ring structure less hydrogen atoms are required than in an alkane, and the general molecular formula for cycloalkanes is CnH2n.
Carbon prefers to have bond angles of 109 degrees and when we look at the shape of a cyclohexane molecule we find a specific type.
Review the 3-D shapes in your text for various cycloalkanes.
The replacement of two or more hydrogen atoms in a cycloalkane by different substituent groups on two or more carbons of the ring can give rise to a unique configurational isomerism. When the substituents are on the same side of the ring this is termed cis, and when on opposite sides it is termed trans.
Review the examples in your textbook.
In the next section on unsaturated hydrocarbons, we will learn that the
carbon to carbon double bond is not free to rotate and when a hydrogen
atom on each of the two carbons is replaced by a different substituent
group, then cis/trans forms are possible.
The key words or phrases from this Lecture Notes are:
- saturated hydrocarbons;
- alkanes;
- cycloalkanes;
- functional groups;
- isomers- structural,
- conformational,
- geometric or cis-trans;
- alkyl;
- IUPAC;
- methane,ethane, propane, butane, pentane, hexane, heptane, octane, nonane, decane;
- molecular shape
- and Valence Shell Electron Pair Repulsion;
- tetrahedron with 109.5 degrees bond angles as in methane;
- sigma covalent bonds via head-to-head overlap of valence electrons;
- SI units where joule replaces calorie and 1 joule = 0.239 calories.