
I. Introduction - Aldehydes and ketones begin the consideration of the superfamily whose members contain the functional group termed the carbonyl ( carbon is SP2 hybridized and double bonded to oxygen, leaving two electrons for bonding to other atoms). This functional group is considered the most important one in organic chemistry, and contains a region rich in electrons (the double bond) and is polar because of the difference in the electronegative value of oxygen and carbon.
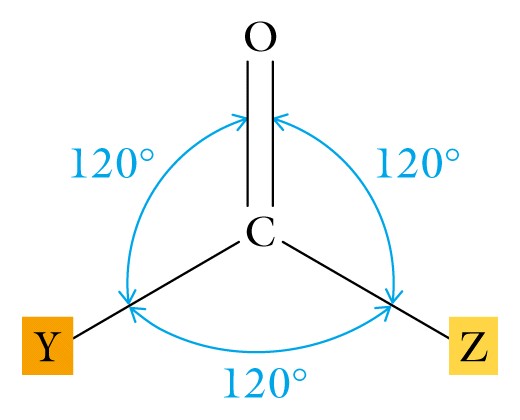
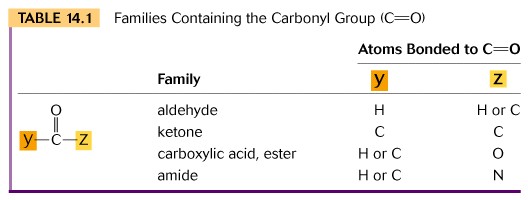
II Aldehydes are characterized by having one of the available electrons on carbon bonded to hydrogen and are named by dropping the (-e) of the alkane name and replacing it with (-al). Since you have learned the first ten alkanes, you now know the first ten aldehydes.
Ketones use both available electrons on carbon to bond to two additional carbon atoms and are named by dropping the (-e) of the alkane and replacing it with (-one).
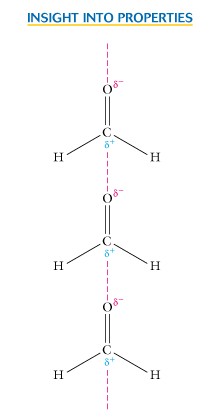
III. The physical properties are influenced by the polarity of the carbonyl group, and since oxygen is a strong electronegative center hydrogen bonding occurs with aldehydes and ketones.
IV. The chemical reactions are very numerous with aldehydes and ketones.
Nucleophilic additions to carbonyl carbon include:
- hydration, acid or base catalyzed;
- addition of alcohols;
- addition of ammonia.
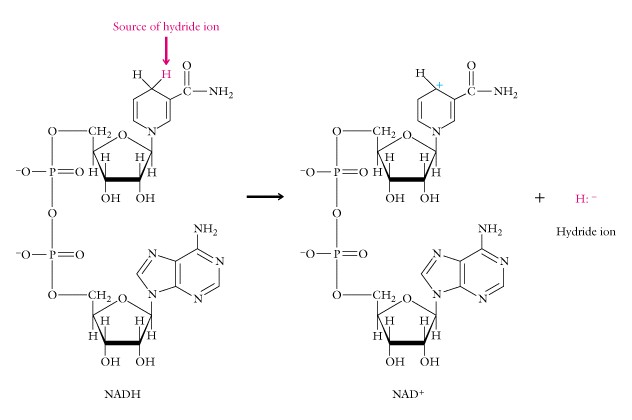
Other reactions are oxidation (ketones are difficult), and reduction (using sodium borohydride, which contains the hydride ion, a hydrogen atom with a pair of electrons and a negative charge).
Still other reactions include
- carbonyl condensations
- hemiacetal
- hemiketal formation of sugars.
The addition of alcohol to an aldehyde or ketone gives the acetal or the ketal i.e. a compound with two (-OR) groups bonded to the same carbon. This addition occurs in two steps and the first one is the hemiacetal or hemiketal. When the two functional groups i.e. carbonyl and hydroxyl occur in the same molecule, as in sugars, an intramolecular reaction occurs producing a heterocyclic ether (furan or pyran) from the nucleophilic addition and a stable first product is formed.
V. Review the structure of the carbonyl functional group. The carbon to oxygen double bond is polarized because of the high electronegativity of oxygen relative to carbon. Since the carbonyl carbon is delta positive it is electrophilic ( a Lewis acid) and attacked by nucleophiles and bases. The carbonyl oxygen is delta negative and is a nucleophilic ( a Lewis base) site and reacts with acids and electrophiles.
Review the mechanism of base catalyzed nucleophilic addition of water and acid catalyzed addition of water. The product is the same but the mechanism is different and illustrates the two major sites of reaction.
VI. An interesting biological product is cyanogenic glycosides found in certain plant structures. Hydrogen cyanide (HCN) is added to the carbonyl group to produce the cyanide derivative. When enzymes cleave this molecule it releases cyanide and insects are discouraged from eating leaves containing these cyanogenic glycosides.
VII. An important biological carbonyl condensation reaction is the addition of acetyl coenzyme A to oxaloacetic acid to produce citric acid of the Krebs Cycle.
The condensation of acetyl coenzyme A molecules is also involved in the biosynthesis of steroids, fats, and other lipids.