

(alternative view)
Thomas H. Mareci, D.Phil.
- Director, Center for Structural Biology, College of Medicine , University of Florida , Gainesville, Florida
- Associate Professor, Department of Biochemistry and Molecular Biology, University of Florida
- Affiliate Faculty, Department of Physics , University of Florida
- Affiliate Faculty, Department of Biomedical Engineering, University of Florida
- Member:
- Publications
- Research Group Home Page
Teaching
- BCH 4024, Lecture Notes and Homework (at UF WebCT Vista)
- BCH 6741, Lecture Notes and Homework (at UF WebCT Vista)
Collaborators
- Doug Anderson, Ph.D., Professor in the Department of Neuroscience, UF
- Steve Blackband, Ph.D., Professor in the Department of Neuroscience, UF
- Bill Ditto, Ph.D., Professor in the Department of Biomedical Engineering, UF
- David Muir, Ph.D., Professor in the Department of Pediatrics, UF
- Baba Vemuri, Ph.D., Professor in the Department of Computer Information Science and Engineering
Research Interest
Our research focuses on the study of fundamental questions of tissue structure and biochemical processes in living systems, which are accessible to study with nuclear magnetic resonance (NMR) techniques. To provide a detailed understanding of the living system, we are examining excised tissue with MR microscopy and spectroscopy. Then these measurements are extended to studies in vivo. This work involves a detailed investigation of biophysical processes at the cellular and molecular level along with the development of NMR measurement and processing methods, and specialized hardware. Our current projects are the following. 1) We are studying blood-spinal-cord barrier and blood-brain barrier disruption, using dynamic contrast enhanced MR imaging in vivo, following trauma. As part of this study, we are modeling the kinetics of lesion development in a longitudinal study of barrier disruption over weeks. 2) Also we are using diffusion tensor imaging to map fiber tracts in highly structured white matter in nervous tissue and have recently extended this procedure to allow fiber tracking in the gray matter of the spinal cord and brain. 3) We are constructing unique RF coils for implantation near the region of interest. These chronically implanted coils are inductively coupled to an external coil during measurements and provide a gain as large as a factor of 4 in signal-to-noise ratio. Because of the gains possible, these coils allow the acquisition of very high spatial resolution MR images and spectra.Research Students
Research students in this program learn the physics of magnetic resonance, gain experience in the operation of NMR instrumentation, learn computer programming for data analysis, and construct RF coils for NMR measurements. Each student designs experiments to acquire and process data, constructs hardware and writes processing algorithms as needed, and fits the data to models of the physiological process under study. In this way the student gains a complete understanding of research procedures from experimental design to modeling the result.Former Students and Staff
Current Research Projects:
We are working on methods to provide a non-destructive evaluation of spinal cord injury, pharmacological neuroprotection, and to follow the process of injury repair. With magnetic resonance images, we are examining the anatomy of the spinal cord in high resolution to provide a unique view of the intact tissue. In particular, we are examining the anisotropy of translational self-diffusion of water in spinal cord tissue to reveal tissue structures. This is significant because the preservation of tissue structure following injury may be a predictor for the success of neuroprotection or repair with tissue transplantation. Recently we have been able to separate intracellular and extracellular water diffusion in order to image these spaces within the spinal cord. In addition, we are able to localize magnetic resonance spectra to a region of interest within the living animal to provide a direct measure of tissue biochemistry (see example spectrum below). Since magnetic resonance is sensitive to molecular structure, magnetic resonance spectroscopy measures an individual resonance for each molecular group. This gives a signature of the chemicals in tissue and therefore direct information about tissue biochemistry, which is essential for a complete understanding of tissue status, particularly following injury, neuroprotection, and/or tissue repair.
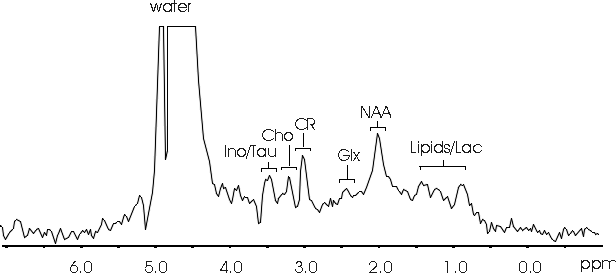
Assignments;
NAA - N-acetyl aspartate compounds, Ino/Tau - inositol/taurine,
Cho - choline-containing compounds, CR - creatine/phosphocreatine,
Glx - glutamine/glutamate, Lipids/Lac - mobile lipids/lacate.
In order to better understanding tissue structure and biochemistry in a living organism, we are examining excised tissue using magnetic resonance. We are studying the structure of intact, excised spinal cords in very high resolution with NMR microscopy (see image below). Using methods we have developed, we are mapping the translational diffusion tensor within a spinal cord to create a complete map of restricted water motion within the tissue. This has lead to the discovery of separate water diffusion in the intracellular and extracellular spaces. Work is currently underway to study injured spinal cords with future work directed at assessing the success of schemes to effect injury repair (e.g. possible cellular transplant). In addition, we are extracting neurochemicals from excised cords to more fully understand the biochemistry of the nervous tissue, which we are able to measure in the living animal using NMR spectroscopy.
Avance 17.6 T, 89 mm bore magnet (Bruker Instruments, Inc.), measured at 750 MHz with 5 mm RF coil
Data processing with IDL (Research Systems, Inc.) and rendered with VoxelView (Vital Images, Inc.)

Multiple-slice spin echo image
TR 2500 ms, TE 16 ms, 8 averages, total time ~43 mins
Field of view; 5 x 5 x 5.76 mm
Matrix 128 x 128 x 48; Resolution, 39 x 39 x 120 microns (voxel size)
External NMR receiver coils do not provide the best coupling to the magnetic resonance signal from small tissues (like the spinal cord), which are located deep in the subject. We have successfully used specially designed coils placed around the tissue of interest in order to increase the measured signal (over a factor of 2 for the spinal cord). The coils are chronically implanted into the living subject without any external connection. The magnetic resonance signal in detected by inductive coupling to an external coil. We are working on an improved coil design to further increase the detected signal strength and to allow the examination of a range of nuclei, hence a broader view of tissue biochemistry.
The resolution of magnetic resonance imaging is determined by the strength of the measured signal and the gradient field produced by specialized hardware. We are developing unique radio-frequency (RF) coils for the measurement of magnetic resonance. Each project (brain, spinal cord) require unique RF coils to optimize the strength of the excised and measured NMR signal. These RF coils allow the measurement of images and spectra from excised tissue in our 14 Tesla and 17.6 Tesla NMR magnet systems and measurements of images and spectra in vivo using our 11.1 Tesla NMR magnet system. At these high magnetic fields, the NMR signal frequency is very high (470 MHz to 750 MHz) and requires careful design, produced by simulation of the electric and magnetic fields, and optimized construction techniques.
The NMR pulse sequences used to acquire data and the processing routines used to produce the images and spectra are available free to investigators who wish to use them for non-commercial purposes. Further information about our methods and hardware designs are published and information available in the following list of journal articles. To obtain information and copies of our software routines, please contact Tom Mareci at thmareci@ufl.edu.
References
Personal
Location of lab and office
Food and Drink (strong coffee and more)
Copyright 2006 University of Florida. All Rights Reserved
Department of
Biochemistry
and Molecular Biology
Box 100245
College of Medicine
University of Florida
Gainesville, FL 32610-0245