PREVENTION
In the past
Smallpox
is the first disease for which control by immunization was developed.  Efforts to
control it began in antiquity. In
Efforts to
control it began in antiquity. In  in
in
Variolation was
met with mixed reviews everywhere it was used; it was effective in some, but  there are many
reports of patients dying of smallpox introduced by variolation, and of
epidemics resulting (Radetsky, 1999). In
1796, Edward Jenner, an English country physician, changed all that. Based on observations that people who milked
cows and contracted cowpox were immune to smallpox, he inoculated a boy with
fluid from a cowpox lesion on a milkmaid’s hand. Two months later, after inoculation with
smallpox fluid, the boy developed no signs of smallpox. Oddly, although Jenner was praised by many
for his pioneering work, he had fierce critics.
Furthermore,
there are many
reports of patients dying of smallpox introduced by variolation, and of
epidemics resulting (Radetsky, 1999). In
1796, Edward Jenner, an English country physician, changed all that. Based on observations that people who milked
cows and contracted cowpox were immune to smallpox, he inoculated a boy with
fluid from a cowpox lesion on a milkmaid’s hand. Two months later, after inoculation with
smallpox fluid, the boy developed no signs of smallpox. Oddly, although Jenner was praised by many
for his pioneering work, he had fierce critics.
Furthermore,  there is some
question as to whether Jenner’s vaccine was actually cowpox; the material he
used initially may have come from horsepox, to which both horses and cows were
susceptible. Nevertheless, cowpox
vaccination gradually became widely accepted and made great inroads in the
coming years towards preventing smallpox.
there is some
question as to whether Jenner’s vaccine was actually cowpox; the material he
used initially may have come from horsepox, to which both horses and cows were
susceptible. Nevertheless, cowpox
vaccination gradually became widely accepted and made great inroads in the
coming years towards preventing smallpox.
Current vaccine
The
vaccine available today is made from live vaccinia virus, which, like variola,
is an  orthopoxvirus;
its use emerged sometime during the 19th century. It is not the cowpox virus Jenner used, and
the circumstances of why and when the vaccination changed are unknown (Fenner
et al., 1988, 278).
orthopoxvirus;
its use emerged sometime during the 19th century. It is not the cowpox virus Jenner used, and
the circumstances of why and when the vaccination changed are unknown (Fenner
et al., 1988, 278).
The Centers for
Disease Control and Prevention (CDC, http://www.cdc.gov/smallpox)
report that historically the modern vaccine has been 95% effective in
protecting people who are vaccinated, and that those receiving the vaccine are
highly immune to the disease for three to five years. After that, immunity steadily decreases, and
protection is questionable after about ten years. Revaccination can extend immunity. Furthermore, although there is no cure for
smallpox, vaccination can prevent or reduce severity of the disease if a person
exposed to the virus is vaccinated within four days of exposure and prior to
the appearance of the rash.
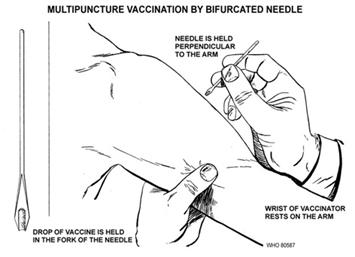
 Administration of the vaccine involves
applying the live virus into the skin with a bifurcated needle that is dipped
into the solution containing the vaccinia.
The skin –typically in the upper arm – is pricked from 15 to 20 times,
just enough to draw a small amount of blood.
The shallow punctures are concentrated into an area of about one square
centimeter. According to the CDC, after
three or four days, if the vaccination “takes,”
Administration of the vaccine involves
applying the live virus into the skin with a bifurcated needle that is dipped
into the solution containing the vaccinia.
The skin –typically in the upper arm – is pricked from 15 to 20 times,
just enough to draw a small amount of blood.
The shallow punctures are concentrated into an area of about one square
centimeter. According to the CDC, after
three or four days, if the vaccination “takes,”  a red, itchy
bump appears which fills with pus and starts to drain, generally at the end of
the first week. During the second week
the lesion dries and scabs over. After
about three weeks the scab falls off, but until that time, since live virus is
administered, the infection can be spread to others if the vaccination site is
not protected. Those who have never
received the vaccine generally have a more severe reaction than people who have
been vaccinated previously.
a red, itchy
bump appears which fills with pus and starts to drain, generally at the end of
the first week. During the second week
the lesion dries and scabs over. After
about three weeks the scab falls off, but until that time, since live virus is
administered, the infection can be spread to others if the vaccination site is
not protected. Those who have never
received the vaccine generally have a more severe reaction than people who have
been vaccinated previously.
Some
complications are associated with vaccination.
 Besides the
typical mild side effects of sore arm, body aches and fever in some cases,
several serious complications can develop.
In fact, the vaccine is contraindicated in people who are immunosuppressed
or have eczema, pregnant or breast feeding women, and children less than one
year of age. When complications do
occur, the WHO reports the following (out of 14 million vaccinations
administered): eczema vaccinatum in
people who have eczema (74 cases, no deaths); vaccinia necrosum (progressive
vaccinia), typically in immmunosuppressed patients, in which the vaccination
lesion progresses and does not heal (11 cases, four deaths); generalized
vaccinia, a rash in healthy individuals (143 cases, no deaths); and
postvaccinial encephalitis, the most serious complication which can result in
paralysis, cerebral impairment, and death in 25% to 35% of cases. Additionally, Esposito and Fenner (2001)
report ocular vaccinia which can result in permanent visual defects.
Besides the
typical mild side effects of sore arm, body aches and fever in some cases,
several serious complications can develop.
In fact, the vaccine is contraindicated in people who are immunosuppressed
or have eczema, pregnant or breast feeding women, and children less than one
year of age. When complications do
occur, the WHO reports the following (out of 14 million vaccinations
administered): eczema vaccinatum in
people who have eczema (74 cases, no deaths); vaccinia necrosum (progressive
vaccinia), typically in immmunosuppressed patients, in which the vaccination
lesion progresses and does not heal (11 cases, four deaths); generalized
vaccinia, a rash in healthy individuals (143 cases, no deaths); and
postvaccinial encephalitis, the most serious complication which can result in
paralysis, cerebral impairment, and death in 25% to 35% of cases. Additionally, Esposito and Fenner (2001)
report ocular vaccinia which can result in permanent visual defects.
