Vioxx
by
Breckon
Pav
Dr.
Bradley
December 5, 2002
History
The selective COX-2 inhibitor Vioxx (rofecoxib) has a relatively short
history on the public market. It
was presented for FDA approval in 1998 by Merck Pharmaceuticals as an
alternative to the traditional pain relievers, non-steroidal anti-inflammatory
drugs (NSAIDs) like Tylenol, Advil, and ibuprofen.
Vioxx was reviewed and approved in 1999 as an effective treatment of
osteoarthritis, menstrual pain (dysmenorrhea), and severe pain in adults (Miceli
1).
Since then, Vioxx has undergone further studies concerning its effects on
the formation of gastrointestinal ulcers and its cardiovascular threats.
Package inserts have changed for better and for worse through the few
years of Vioxx’s popularity. However,
the bottom line is that rofecoxib offers powerful pain relief without the
typical NSAID risk of GI tract infection and ulcers.
Chemical Properties
Rofecoxib has a molecular weight of 314.36 amu, a structure of C17H14O4S,
and is chemically labeled “4-[4-(methylsulfonyl)phenyl]-3-phenyl-2(5H)-furanone.”
The pill color ranges from white to yellowish.
Inactive ingredients in Vioxx are croscarmellose sodium, hydroxypropyl
cellulose, lactose, magnesium stearate, microcrystalline cellulose, yellow
ferric oxide, and red ferric oxide (in 50 mg pills); oral suspensions include citric acid monohydrate (·H2O), sodium citrate dihydrate (·2H2O), sorbitol solution, strawberry flavor, xanthum gum, purified water,
sodium methylparaben (0.13% as preservative), and sodium propylparaben (0.02% as
preservative) (Description 1).
The
Solubility of Rofecoxib
|
Water
|
Acetone
|
Methanol/Isopropyl
Acetate
|
Ethanol
|
Octanol
|
|
Insoluble
|
Slightly
soluble
|
Slightly
soluble
|
Very
slightly soluble
|
Insoluble
|
Rofecoxib falls into a
category of NSAID drugs called COX-2 inhibitors. To understand the nature and mechanisms of Vioxx, it is
necessary to first examine this enzyme and its functions.
Cyclooxygenase
The enzyme
cyclooxygenase (COX) was first isolated in 1979, but it was later discovered
that COX existed in two forms: COX-1
and COX-2 (Bridges 1). COX enzymes
(also called prostaglandin endoperoxide synthase enzymes) aid in the synthesis
of prostaglandins (PGs), a type of fatty acids associated with smooth muscle
contraction. Prostaglandins, when
existing in a certain isoform (H2), can cause irritation to an
inflammation site. The synthesis of
these undesirable prostanoids (prostaglandins, thromboxanes, and others) occurs
in the cell membrane, and is catalyzed by COX-2. COX-2 enzymes congregate at inflamed sites, intensifying the
painful effect by producing PGs. It
was therefore concluded that shutting down the enzyme pathway (COX) used to
create PGs could reduce and even eliminate their effect on inflamed areas.
 The two
COX enzymes exist in different concentrations throughout an organism. At inflammation sites, COX-2 is much more abundant.
Since COX-1 helps produce prostaglandins that protect the stomach lining,
it is present in larger quantities in the GI tract.
Because typical NSAIDs are not selective when targeting enzymes, they end
up shutting down prostaglandin synthesis everywhere in the body.
This results in a greater risk of GI tract infection and ulceration,
inciting research to develop a drug that inhibits only COX-2, the more
pro-inflammatory of the two enzymes.
The two
COX enzymes exist in different concentrations throughout an organism. At inflammation sites, COX-2 is much more abundant.
Since COX-1 helps produce prostaglandins that protect the stomach lining,
it is present in larger quantities in the GI tract.
Because typical NSAIDs are not selective when targeting enzymes, they end
up shutting down prostaglandin synthesis everywhere in the body.
This results in a greater risk of GI tract infection and ulceration,
inciting research to develop a drug that inhibits only COX-2, the more
pro-inflammatory of the two enzymes.
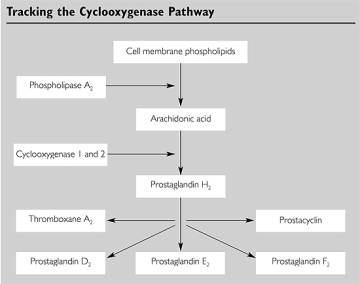
As
shown in Figure 1, both COX enzymes catalyze a critical step in similar
reactions: Converting free membrane arachidonic acid to a prostaglandin.
External physiologic stimuli (e.g. tissue damage) can activate
phospholipase A2, which cuts arachidonic acid from the membrane
(Selective 1). COX enzymes can then
interact with the free membrane arachidonic acid and convert it to prostaglandin
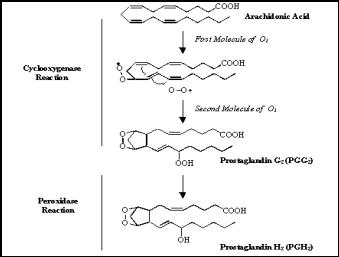
form. This is accomplished by
adding O2 to the molecules in a two-step process.
These steps are outlined in Figure 2.
This unstable prostaglandin must immediately be changed into more stable
molecules (thromboxanes, stable prostaglandins, prostacyclin).
The enzyme peroxidase is conveniently coupled to cyclooxygenase in the
membrane to serve this exact purpose. When
a prostaglandin H2 is formed from the PGG2, it is free to
enter the cytoplasm, where it is permanently stabilized by another enzyme.
From there,
 the
prostaglandin acts as a signaling molecule to the surrounding cells that have
prostaglandin receptors (which are G-protein-linked receptors).
When a PG interacts with a receptor, the cell is stimulated (through the
adenylyl cyclase
the
prostaglandin acts as a signaling molecule to the surrounding cells that have
prostaglandin receptors (which are G-protein-linked receptors).
When a PG interacts with a receptor, the cell is stimulated (through the
adenylyl cyclase
pathway)
to contract.
Until
quite recently, COX enzymes were inhibited by non-steroidal anti-inflammatory
drugs (or NSAIDs). These drugs,
such as ibuprofen or Tylenol, boasted pain relief to inflamed or injured areas.
However, since NSAIDs inhibited all COX enzymes without selectivity, the
COX-1 enzyme could not perform its proper function of catalyzing the synthesis
of protective PGs for the stomach and kidneys.
Thus, when taken in high doses, NSAIDs generated unwanted side effects of
GI tract infection and even stomach and intestinal ulcers.
To get enough pain relief for rheumatoid arthritis, achy joints, or
post-surgical pain, NSAIDs would have to be taken in amounts high enough to
invoke gastric ulcers. Therefore,
NSAIDs did not adequately solve the COX problem.
The
need for an effective and selective COX-2 inhibitor resulted in the research
production of two similar drugs: celecoxib
(Celebrex) and rofecoxib (Vioxx). Although pharmacies began distributing celecoxib first, with
an impressive 375-fold selectivity rate for COX-2, rofecoxib bragged a much
higher rate of selectivity (Kaplan-Malchis 27).
Yet both attempt to accomplish the same goal of inhibiting COX-2 only.
Rofecoxib
(and similar COX-2 inhibitors) is extremely selective in its enzyme inhibitions,
with an incredible selectivity ratio of COX-2 to COX-1 greater than 800:1 (27).
Since COX-2 exists in high concentrations at the site of inflammation,
the drug’s effects are detected mostly in this area.
Pro-inflammatory prostaglandins are prevented from developing,
significantly reducing swelling and acute pain at potential inflammatory sites.
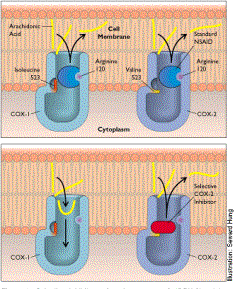 Rofecoxib
is able to accomplish its task by taking advantage of a key difference in amino
acid sequences on COX-1 and COX-2. At a specific spot where COX-1 has
isoleucine, COX-2 carries a valine. The presence of the valine allows the
selective COX-2 inhibitor to bind only to that site on COX-2.
Rofecoxib searches for this valine in a COX enzyme and binds to the site
only when it is present. In this
way, the drug can selectively inhibit COX-2 from producing PGs.
Rofecoxib
is able to accomplish its task by taking advantage of a key difference in amino
acid sequences on COX-1 and COX-2. At a specific spot where COX-1 has
isoleucine, COX-2 carries a valine. The presence of the valine allows the
selective COX-2 inhibitor to bind only to that site on COX-2.
Rofecoxib searches for this valine in a COX enzyme and binds to the site
only when it is present. In this
way, the drug can selectively inhibit COX-2 from producing PGs.
Administration
of Rofecoxib
Vioxx is taken orally in doses of 12.5 mg, 25 mg, or 50 mg, with or
without food. The 50 mg tablet is seldom used, as doctors only recommend a
maximum of 25 mg per day. Since
rofecoxib interacts with certain other drugs, it should not be taken with
methotrexate, warfarin, rifampin, ACE inhibitors, or lithium (Jillard 1).
Vioxx is 93% absorbed by the body, and excreted over 99% of the time by
urine (Clinical 1). It can also be given in oral suspensions of 12.5 mg/mL or 25
mg/mL (Jillard 1).
Side
Effects
Initially, Vioxx inserts contained warnings identical to traditional
NSAIDs regarding GI tract infection and ulceration until a survey showed some
impressive results. Both U.S. and
international studies of the connection between Vioxx and endoscopic
gastroduodenal ulcers, a 25 mg dose exhibited about the same effect on stomach
lining as a placebo (sugar pill). Ibuprofen
understandably had nearly three times as many ulcer cases as the placebo.
It can be concluded, therefore, that due to the selective nature of
rofecoxib, Vioxx does not interact with COX-1 nearly as much as Ibuprofen.
Tables 2 and 3 show the results of these studies.
|
Endoscopic
Gastroduodenal Ulcers at 12 weeks
U.S. Study
|
|
Treatment
Group
|
Number
of Patients with Ulcer/
Total Number of Patients
|
Cumulative
Incidence Rate
|
Ratio
of
Rates
vs. Placebo
|
95%
Cl
on
Ratio of Rates
|
|
Placebo
|
11/158
|
9.9%
|
--
|
--
|
|
VIOXX
25 mg
|
7/186
|
4.1%
|
0.41
|
(0.16,
1.05)
|
|
VIOXX
50 mg
|
12/178
|
7.3%
|
0.74
|
(0.33,
1.64)
|
|
Ibuprofen
|
42/167
|
27.7%
|
2.79
|
(1.47,
5.30)
|
|
*by
life table analysis
|
<http://www.healthandage.com/html/res/pdr/html/52402550.htm>
|
|
Endoscopic
Gastroduodenal Ulcers at 12 weeks
Multinational Study
|
|
Treatment
Group
|
Number
of Patients with Ulcer/
Total Number of Patients
|
Cumulative
Incidence Rate
|
Ratio
of
Rates
vs. Placebo
|
95%
Cl
on
Ratio of Rates
|
|
Placebo
|
5/182
|
5.1%
|
--
|
--
|
|
VIOXX
25 mg
|
9/187
|
5.3%
|
1.04
|
(0.36,
3.01)
|
|
VIOXX
50 mg
|
15/182
|
8.8%
|
1.73
|
(0.65,
4.61)
|
|
Ibuprofen
|
49/187
|
29.2%
|
5.72
|
(2.36,
13.89)
|
|
*by life table
analysis
|
These studies show the frequency in occurrence of intestinal ulcers over
a twelve-week period for individuals who took these drugs.
For the few cases that resulted in ulcers, these side effects can be
traced back to the fact that rofecoxib is not exclusively selective of COX-2,
and in fact, some COX-1 enzymes are inhibited in the process.
This causes a rare incident of gastroduodenal ulceration.
Based on a clinical study of the negative effects of Vioxx compared with
Ibuprofen,
|
Side
Effects Occurring in ³2% of
Patients Treated with Rofecoxib
|
|
|
Placebo
|
Rofecoxib
12.5 or 25 mg
daily
|
Ibuprofen
2400 mg
daily
|
Diclofenac
150 mg
daily
|
|
|
(N
= 783)
|
(N
= 2829)
|
(N
= 847)
|
(N
= 498)
|
|
General
Abdominal Pain
Asthenia/Fatigue
Dizziness
Influenza-Like Disease
Lower Extremity Edema
Upper Respiratory Infection
|
4.1
1.0
2.2
3.1
1.1
7.8
|
3.4
2.2
3.0
2.9
3.7
8.5
|
4.6
2.0
2.7
1.5
3.8
5.8
|
5.8
2.6
3.4
3.2
3.4
8.2
|
|
Cardiovascular
System
Hypertension
|
1.3
|
3.5
|
3.0
|
1.6
|
|
Digestive
System
Diarrhea
Dyspepsia
Epigastric Discomfort
Heartburn
Nausea
|
6.8
2.7
2.8
3.6
2.9
|
6.5
3.5
3.8
4.2
5.2
|
7.1
4.7
9.2
5.2
7.1
|
10.6
4.0
5.4
4.6
7.4
|
|
EENT
Sinusitis
|
2.0
|
2.7
|
1.8
|
2.4
|
|
Musculoskeletal
System
Back Pain
|
1.9
|
2.5
|
1.4
|
2.8
|
|
Nervous
System
Headache
|
7.5
|
4.7
|
6.1
|
8.0
|
|
Respiratory
System
Bronchitis
|
0.8
|
2.0
|
1.4
|
3.2
|
|
Urogenital
System
Urinary
Tract Infection
|
2.7
|
2.8
|
2.5
|
3.6
|
Diclofenac,
and a placebo, rofecoxib was proven a relatively safe and effective drug for use
in pain management. As shown in
Table 4, Vioxx behaved more like a placebo in most cases than a drug.
About 25% of Vioxx recipients reported some type of side effect, with
only about 5% of the total patients contracting serious health problems from the
drug (Selective 1). One of the most common side effects among patients was
diarrhea (6.5%). Several isolated
cases of GI tract infection, ulceration, kidney failure, or cardiovascular
complications. All of these side
effects occur due to minute COX-1 inhibition.
Along with the severe side effects, Vioxx also causes some typical
medicinal side effects: allergic
reactions, skin reactions, liver problems (nausea, tiredness, itching,
tenderness in the right upper abdomen, and flu-like symptoms), and other minor
and rare incidents of anxiety, confusion, depression, hair loss, hallucinations,
increased levels of potassium in the blood, low blood cell counts, palpitations,
pancreatitis, tingling sensation, unusual headache with stiff neck (aseptic
meningitis), vertigo. Table 4 shows
an exhaustive list of possible side effects for rofecoxib compared with
Ibuprofen, Diclofenac, and a placebo.
Conclusion
Vioxx has a promising future as a valid treatment for arthritic symptoms,
joint pain, and post surgical recovery. Its
arrival in pharmacies is definitely a welcome relief and alternative for
traditional non-selective NSAIDs. When
taken in small enough doses, rofecoxib can effectively inhibit COX-2 enzymes,
eliminating the synthesis of adverse prostaglandins, without seriously affecting
the recipient’s health.
Works
Cited
“Adverse Reactions:
Vioxx Prescribing Information.” Merck
& Co., Inc. 26 Nov 2002. <http://www.vioxx.com/vioxx/product_info/pi/reactions.html>
Bridges, Alan J.
“COX-2 Inhibitors.” Spine-Health,
1999. 26 Nov 2002. <http://www.spine-health.com/topics/conserv/cox/cox03.html>
“Clinical Pharmacology:
Vioxx Prescribing Information.” Merck
& Co., Inc. 26 Nov 2002.
<http://www.vioxx.com/vioxx/product_info/pi/clin_pharm.html>
“Description:
Vioxx Prescribing Information.” Merck
& Co., Inc. 26 Nov 2002.
<http://www.vioxx.com/vioxx/product_info/pi/description.html>
Kaplan-Malchis, Barbara.
“A Quest for Safer NSAIDs: Focus
on the Selective COX-2 Inhibitors.” Home
Health Care Consultant. June
2000: 25-30.
26 Nov 2002. <http://www.mmhc.com/hhcc/articles/HHCC0006/kaplanmachlis.html>
Jillard, Nancy.
“Pain and Rheumatoid Arthritis: An
Update.” Drug Topics.
Medical Economics: Montvale,
2000. 26 Nov 2002. <http://www.drugtopics.com/be_core/search/show_article_search.jsp?searchurl=/be_core/content/journals/d/data/2000/0403/dce04a.html&navtype=d&heading=d&title=Pain+%26amp%3B+Rheumatoid+Arthritis%3A+An+Update>
McGriff, Nayahmka.
“Management of pain in rheumatoid arthritis.”
Drug Topics. Medical
Economics: Montvale, 2001. 26
Nov 2002. <http://www.drugtopics.com/be_core/search/show_article_search.jsp?searchurl=/be_core/content/journals/d/data/2000/0403/dce04a.html&navtype=d&heading=d&title=Pain+%26amp%3B+Rheumatoid+Arthritis%3A+An+Update>
Miceli, David.
Physician’s Desk Reference.
53rd-55th editions: 1999-2002.
26 Nov 2002. <http://www.stevensjohnsonsyndrome.com/vioxx.htm>
“Selective COX-2
Inhibitors.” The Drug Monitor.
26 Nov 2002. <http://www.home.eznet.net/~webtent/coxi.html>
 The two
COX enzymes exist in different concentrations throughout an organism. At inflammation sites, COX-2 is much more abundant.
Since COX-1 helps produce prostaglandins that protect the stomach lining,
it is present in larger quantities in the GI tract.
Because typical NSAIDs are not selective when targeting enzymes, they end
up shutting down prostaglandin synthesis everywhere in the body.
This results in a greater risk of GI tract infection and ulceration,
inciting research to develop a drug that inhibits only COX-2, the more
pro-inflammatory of the two enzymes.
The two
COX enzymes exist in different concentrations throughout an organism. At inflammation sites, COX-2 is much more abundant.
Since COX-1 helps produce prostaglandins that protect the stomach lining,
it is present in larger quantities in the GI tract.
Because typical NSAIDs are not selective when targeting enzymes, they end
up shutting down prostaglandin synthesis everywhere in the body.
This results in a greater risk of GI tract infection and ulceration,
inciting research to develop a drug that inhibits only COX-2, the more
pro-inflammatory of the two enzymes.
 the
prostaglandin acts as a signaling molecule to the surrounding cells that have
prostaglandin receptors (which are G-protein-linked receptors).
the
prostaglandin acts as a signaling molecule to the surrounding cells that have
prostaglandin receptors (which are G-protein-linked receptors). Rofecoxib
is able to accomplish its task by taking advantage of a key difference in amino
acid sequences on COX-1 and COX-2. At a specific spot where COX-1 has
isoleucine, COX-2 carries a valine. The presence of the valine allows the
selective COX-2 inhibitor to bind only to that site on COX-2.
Rofecoxib
is able to accomplish its task by taking advantage of a key difference in amino
acid sequences on COX-1 and COX-2. At a specific spot where COX-1 has
isoleucine, COX-2 carries a valine. The presence of the valine allows the
selective COX-2 inhibitor to bind only to that site on COX-2.