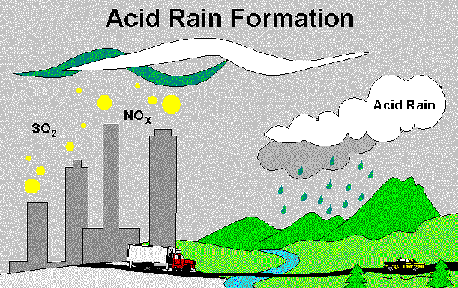
Acid
Rain Formation
Acid rain is caused by emissions
of sulfur dioxide and nitrogen oxides. Natural sources of these gases
do exist; however, more than 90% of the sulfur and 95% of the nitrogen
emissions are from human activities. The use of coal for electricity,
fuel for vehicles, and many other
factors contribute to the pollution. Once these toxins are in
the air, they are converted to nitric and sulfuric acid by reacting with
water, oxygen, and oxidants. The basic reactions for each follow:
SO2 + 2OH --- H2SO4 (sulfuric acid)
NO2 + OH --- HNO3 (nitric acid)
Both of these acids are easily dissolved in water.
Consequently, acidic water returns to Earth in the form of rain snow or
fog. The sulfur dioxide and nitrogen oxide emissions can also chemically
transform into sulfur and nitrogen salts. In this state, they are
deposited as "dry" acid rain in the form of gases or particles. This
"dry" rain causes the same type of damage as regular acid rain, but it
can be transferred hundreds of miles by prevailing winds.