Combustion characterization of fluorinated polymers
Introduction
This is a description of a project sponsored by USAF through New Era technology where we characterized the combustion of several different fluorinated polymers through heating samples in a test cell mounted in an FTIR spectrometer. The testcell could be filled with argon or dry air to investigate the impact of the surrounding gas on the decomposition and oxidation of the polymer samples. The test cell could also be heated to reduce the problem of water vapor condensating on the walls of the stainless steel test cell over time.
Fluorinated Polymers
Fluorine is the most electronegative of the elements of the periodic table, making it a powerful oxidizing agent. As such, fluorine gas, F2, is extremely corrosive and toxic, reacting violently with most substances forming fluorides, or in the case of many hydrogen-containing compounds such as water, hydrofluoric acid. This extreme reactivity means that fluorine, as its fellow halogens, can successfully outcompete oxygen for the fuel radicals produced during combustion, preventing further reactions and explaining its use as a component in many fire-fighting compounds. Due to the greenhouse effect and ozone-layer depletion potential of many halogenated compounds (e g CFCs), a great deal of research has been directed towards understanding the chemistry of these compounds under combustion conditions. The present work is a continuation of this work, primarily aiming at understanding the decomposition and combustion of fluorinated polymers which may be used to deliver reactive fluorine and hydrofluoric acid in a non-reactive package.
Experimental setup and procedure
A milligram size sample of the fluorinated polymer sample to be tested was inserted into the test cell, coating a nickel-chromium or platinum glow spiral. The test cell is inserted into the sample compartment of a Nicolet Magna-IR 560 FT-IR spectrometer, as shown in the photograph below, and the glow spiral leads are connected to a power supply capable of supplying up to 3A through the wire that has a diameter of 0.25 mm and a resistance of ~2.5 ohm. The cell and spectrometer is purged using breathing grade air or industrial grade argon, reducing the levels of in particular water and carbon dioxide. While purging, the background spectra in the 900-4200 cm-1 range are monitored every few minutes on a computer connected to the spectrometer. Once the absorption level is found acceptably low, the test cell is sealed off with a pressure transducer and thermocoule inserted into the main body. Thereafter data acquisition is started and spectra are acquired with an approximate spacing of ~10 s. The power is switched on and the combustion progress is monitored for several minutes as spectra are acquired at longer and longer intervals. After approximately 10 minutes, steady state is reached and the test is concluded. Post-processing of the spectral data (typically ~20 spectra with 2 cm-1 resolution) involves classic least squares comparison to a data base of 25 species expected to be found among the products. Before this, a baseline correction has been performed through a polynomial fit through low-absorption regions of the collected spectra. The results are time traces of quantitative species concentrations, as well as of the temperature and pressure in the cell.

Examples of results
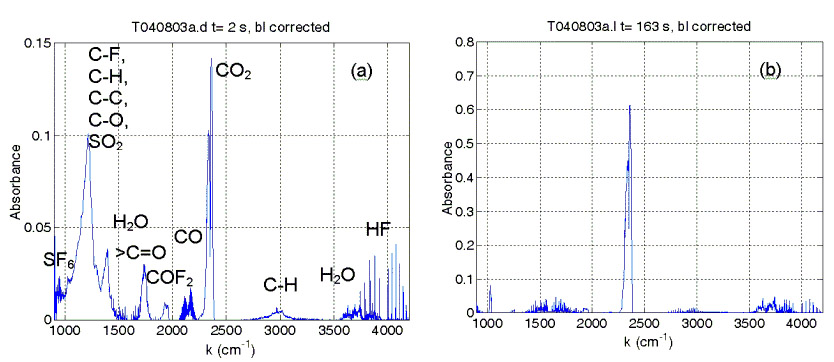
Spectra acquired at different times after heating was applied to a sample of the GB1 polymer. The left spectrum also shows identified absorption features of different compounds and chemical bonds just after application of heating.
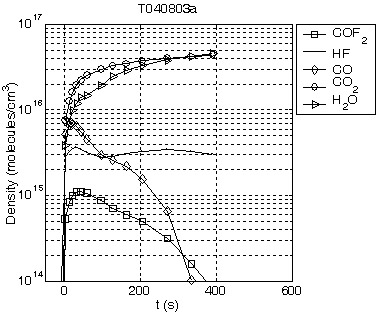
Time traces of the concentrations of major species as heat is applied to a sample of GB1.
More details of these experiments can be found in these publications:
 Gustavsson, J. P. R., Segal, C., "Combustion and thermal decomposition of fluorinated polymers", Journal of Combustion Science and Technology, 178 (12), pp. 2097-2114, 2006.
Gustavsson, J. P. R., Segal, C., "Combustion and thermal decomposition of fluorinated polymers", Journal of Combustion Science and Technology, 178 (12), pp. 2097-2114, 2006.
 Gustavsson, J P R, Lerma, N, Segal, C, "Combustion Characterization of Fluorinated Polymers", AIAA Paper 2004-3883, 40th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Ft Lauderdale, Florida, July 11-14, 2004.
Gustavsson, J P R, Lerma, N, Segal, C, "Combustion Characterization of Fluorinated Polymers", AIAA Paper 2004-3883, 40th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Ft Lauderdale, Florida, July 11-14, 2004.
Links
Back to the UF research page




 Gustavsson, J. P. R., Segal, C., "Combustion and thermal decomposition of fluorinated polymers", Journal of Combustion Science and Technology, 178 (12), pp. 2097-2114, 2006.
Gustavsson, J. P. R., Segal, C., "Combustion and thermal decomposition of fluorinated polymers", Journal of Combustion Science and Technology, 178 (12), pp. 2097-2114, 2006.
 Gustavsson, J P R, Lerma, N, Segal, C, "Combustion Characterization of Fluorinated Polymers", AIAA Paper 2004-3883, 40th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Ft Lauderdale, Florida, July 11-14, 2004.
Gustavsson, J P R, Lerma, N, Segal, C, "Combustion Characterization of Fluorinated Polymers", AIAA Paper 2004-3883, 40th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Ft Lauderdale, Florida, July 11-14, 2004.