There are three physical states that matter can have (four counting plasma, but that is typically only found under extreme temperatures like those in the Sun). These are: solid, liquid, and gas. Water is readily found in each of these states on the Earth.
The picture to the right shows all three states, a solid iceberg, liquid ocean, and gaseous clouds (technically, clouds are liquid water, but actual water vapor is invisible so we so we will assume that there is plenty of water vapor in the air if we can see clouds).
The rest of this page will study the physical properties of water in more depth.

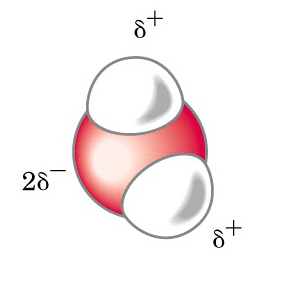
The picture to the left shows a single water molecule and the relative charges that the different parts of the molecule possess. It will not be necessary to know anything about these charges other than the fact that they are responsible for many of the unique characteristics of water. Because the molecule is not bounded to any other water molecules, it is in the water vapor, or gaseous state.
Water in this state is invisible (due to its extremely small size). Gaseous water molecules freely move past each other and have very little interaction at all. In order to remain in the gas state, water molecules must possess a lot of energy. This is why cold things like a glass of cold liquid will get covered in condensation. As water vapor hits the cold glass, the glass will absorb some of the energy that the water molecules have. This loss of energy prevents the water molecules from breaking free of other nearby water molecules and they hydrogen bond, as seen below, and form liquid water.
Gasses all share common physical properties. Gasses will change shape to fit the container that they are put into. This isn't easy to see as gasses are typically invisable, but you can indirectly see it when you fill up a balloon. Balloons apply force upon the gas within equally on all sides and are therefore round without hard edges. Gasses can and readily do change volume. As pressure is applied to a volume of gas, that volume will reduce and the gas will get denser. Eventually, gasses are forced to liquefy if placed under enough pressure.
The picture to the right shows water in the liquid state. Notice that the water molecules are no longer touching each other as they had been in the solid state. However, they are still held close together by Hydrogen bonds. These bonds are weak enough to allow the molecules to move past each other, unlike the bonds in ice as seen below.
Liquid compounds will flow and conform to the shape of the container that they are placed into. This is apparent every time you pour yourself a glass of orange juice from a cardboard container. The orange juice goes from the square shape of the carton to the round, cylindrical shape of the glass.
Liquids also resist changing volume. If you apply pressure to liquids, they do not become denser. This property is utilized in hydraulic lifts.


The picture to the left shows a collection of water molecules arranged in an ice crystal. This is water in its solid state.
Solid compounds do not change their shape to fit a container, nor will their volume change. What this means is obvious, if you put a rock into a bucket, it stays at its original shape, it doesn't change and flow over the bottom to fill up the container. And if you apply pressure to a solid, its volume won't change and it won't become denser. Solids simply shatter when you try to compress them.
Ice is a somewhat special solid because unlike most solids, ice can actually flow, albeit very slowly. Another solid with this characteristic, the ability to flow, is glass. Technically, both ice and glass are liquids, but since their rate of flow is so small we will consider them to have the same properties as other solids.
The particles in ice are specifically ordered, as you can see in the picture. Water in this form takes up more space than it does in liquid form (this is why full soda or milk bottles will balloon out in the freezer or break if they are made of glass). In other words, ice is less dense than liquid water. This change in volume is another property that sets water apart from solids. For most compounds, the solid form will take up less space (is more dense) than the liquid form. This difference in densities allows ice to float on water. This has been crucial to the survival of aquatic communities.